
Chitosan for metabolism -
The levels of fasting serum insulin and leptin were tested by the Mouse leptin insulin kits Bio-Swamp, China. Serum total cholesterol, triglycerides, low-density lipoprotein cholesterol and high-density lipoprotein cholesterol were determined by the corresponding kits Jiancheng, China.
The amount of short-chain fatty acids in the cecum of mice was tested by the Elisa kit Jianglai, China. Tissue sections 5 μm were then stained with haematoxylin and eosin HE and visualized under a microscope Olympus BX51, Japan.
The sizes of the adipocytes were measured by ImageJ National Institutes of Health, USA. The tissue was first cut into small pieces with scissors and then ground with a grinder JXFSTPRP series automatic sample grinding machine, Jingxin company, Shanghai, China according to the instruction manual.
Total RNA was extracted with TRIzol® CWBIO, China according to the manufacturer's instructions. The TRIzol® reagent can extract RNA from tissues and cells efficiently, and contains a protocol for purification of sample RNA from human, animal, plant or bacterial sources.
The TRIzol® reagent maintains RNA integrity by inhibiting RNase activity during sample homogenization, while destroying cells and dissolving cellular components. The total RNA separated by TRIzol® reagent does not contain protein and DNA contamination.
The mRNA concentration was determined using an ultramicrospectrophotometer Thermo, Waltham, MA, USA. Equal amounts of mRNA from each mouse were pooled to normalize individual differences, and mRNA was reverse-transcribed to cDNA using a HiFiScriptcDNA Synthesis Kit CWBIO.
Quantitative real-time PCR was performed with UltraSYBR Mixture CWBIO on a Light Cycler 96 Real-Time PCR System Roche, Switzerland , and amplified for 45 cycles of denaturation at 95°C for 10 s, annealing at 60°C for 30 s and extension at 72°C for 32 s.
The housekeeping gene GAPDH is used as an internal reference for gene expression, and the primers used for amplification were shown in Supplementary Table 2. The fecal samples were collected from the four groups of mice on days 0, 7, 28, and 56, and cecal content samples were collected from each mouse when the mice were sacrificed.
All PCRs were performed with a 30 μL reaction volume containing 15 μL Phusion® high-fidelity PCR master mix New England Biolabs , 0. Thermal cycling included initial denaturation at 98°C for 1 min, 30 cycles of denaturation at 98°C, annealing at 50°C for 30 s, and extension at 72°C for 30 s.
The final stage was 72°C for 5 min. Then, the mixed PCR products were purified using GeneJETTM Gel Extraction Kit Thermo Scientific.
The V3-V4 region of 16S rRNA was sequenced on the Ion S5 sequencing platform Novogen Co. The average sequencing depth of each sample was 66, reads. Bioinformatical processing was performed with Qiime v2 and Usearch.
The analysis in this study adopted the methods of relative abundance to normalize the compositional and sparse data, and Permutation Test was used to calculate differential abundance among groups.
Spearman correlation analysis was employed to analyze the relationship between related indicators. All data are expressed as the mean ± standard deviation SD unless otherwise specified.
Statistical tests were performed by GraphPad Prism software version 6, MacKiev Software, Boston, MA, USA and R software version 3. To determine the effect and safety of COS under the circumstance of high-fat and low-fat diets, the body weights and food intakes of mice in each group were monitored from 0 to 56 days.
The results showed the average weight gain of the HC group was significantly slower than H group Figure 1A , yet the food intake exhibited no divergence among the four groups Figure 1B.
We then calculated the energy intake, which showed similarity between the H and HC groups and higher than L and LC groups Figure 1C. COS markedly reduced the weight gain per unit of dietary intake in high-fat diet groups, while no distinction was observed in the low diet groups Figure 1D.
The body fat rate evaluation showed that the fat weight and rate of the low-fat diet group were greatly lower than those of the high-fat diet group, while the HC group was clearly lower than that of the H group in the high-fat diet groups Figures 1E,F.
Additionally, the average liver weight of the H group was found higher than HC groups and L group Figure 1G.
The results indicated that the Epi-WAT cell area of the low-fat feeding groups was significantly lower than that of the high-fat feeding groups, and COS supplementation significantly reduced the area of Epi-WAT cell in the high-fat diet group Figure 1I. The size of fat cells also indicated constitutive variation of H to HC and L groups Figure 1J.
These results indicate that COS supplementation could improve fat cell hypertrophy caused by a high-fat diet. Figure 1. Effects of COS on Body weight and liver fat.
Groups: Group L low-fat diet group, fed with 4. When the mice were given a high-fat diet for a period of time, metabolic dyslipidemia might be induced; the amount of blood total cholesterol TCHO demonstrated a variation between the L and H groups Figure 2A. However, the shift in some blood lipid metabolites was not significant, such as low and high density lipoprotein cholesterol LDL-C and HDL-C , although their average values exhibited fluctuations Supplementary Figures 1A—D.
Meanwhile, the addition of COS did alleviated dyslipidemia; the level of TCHO increased, while the levels of HDL-C increased accordingly Supplementary Figures 1A—D. These results indicate that COS can restore certain fat parameters disorders induced by high-fat diet.
Next, the blood glucose deviation instigated by high-fat diet was restored to normal levels by COS Figures 2B,C , whereas the concentrations of serum triacylglycerols TGs and leptin showed no variation among the four groups Supplementary Figures 1A,D.
The fasting blood glucose and insulin levels were further tested. The blood glucose of the H group was found to be significantly higher than that of the L group, while its level in the HC group was restored to be comparable to that of L group Figure 2D. Similarly, the fasting insulin level of H group was also significantly divergent from that of COS-treated HC group and low-fat fed L group Figure 2E ; the HOMA-IR results indicated insulin resistance level was also significantly higher in the H group than in the L group and the HC group Figure 2F.
It is worth noting that the above-mentioned items showed no variation between the L and LC groups, indicating that COS may not affect the energy absorption and metabolism of mice in the case of low-fat diet.
Figure 2. Blood glucose, insulin and physiological metabolites analysis. TCHO A , Glucose B , Fasting glucose C,D , Fasting insulin E and insulin resistance F , HOMA-IR levels of different groups. To explore the role of intestinal microbiota in COS-induced recovery of obesity and metabolite deviation, 16S rRNA analysis was performed toward the fecal samples obtained at day 0, 7, 28 and 56; and the caecal microbiota of day 56 was also tested.
Supplementary Figure 2 showed the changes of α-diversity indexes after COS treatment. The rarefaction curve indicated that all samples tended to be saturated, suggesting the OTUs covered most of the bacterial species in the mouse intestine.
The Shannon indices showed that the α-diversities of the groups with COS were lower than the corresponding control groups, and the p -value decreased gradually over time, implying that COS may induce reduction of within-sample microbial diversity Supplementary Figure 3A.
The Principal coordinate analysis PCoA results exhibited that all dots of COS fed groups LC and HC got more separated from their corresponding control groups L and H as time increased Figure 3A.
The distance boxplot of Bray Curtis exhibited the enhancement of between-sample microbial diversity of intestinal microbiota concomitant with the consumption of COS Figure 3B.
The p -values were 0. Figure 3. Structure of the gut microbiome. A PCOA analysis of the fecal microbiota; B Bray Curtis distance of the fecal microbiota; C Top 10 dominant fecal microbes over time in each group; D Heatmap of differential fecal species at the same time point; E PCOA analysis of the cecum microbiota; F Bray Curtis distance of the cecum microbiota; G Top 10 dominant fecal microbes over time in each group; H Heatmap of differential cecum microbes at the same time point.
Then the compositions of fecal bacteria were interrogated, the result revealed the number of phylum in mice intestine remained unchanged after COS treatment; and this phenomenon was observed in all 7, 28, and 56 d groups.
At the genus level, the number of genera in the fecal microbiota decreased after COS treatment, LC and HC decreased by 52 and 32 genera, respectively, compared with their controls; but at 7 and 28d time points, the HC groups contained more genera than the H groups.
Similarly, COS also induced less Lactobacillus in the low-fat diet groups, but genus Bacteroides increased together with Parabacteroides in LC group.
The changes in abundance at the level of genus were also verified in the Heatmap of each genus Supplementary Figure 3C. Similar to genus level, the decrease in the number of species in mice feces occurred in the 56th day group; but in the 7th and 28th day groups, the HC group contained more species than the H group.
Generally, COS induced more Clostridium paraputrificum and Clostridium ramosum in the high-fat diet groups, while the content of Clostridium cocleatum decreased Figure 3C. In the low-fat diet groups, Parabacteroides goldsteinii and Bacteroides uniformis enhanced their abundance in LC group.
The changes in abundance at the level of species were also verified in the Heatmap of each species Figure 3D and Supplementary Figure 3D. It is worth noting that the abundance of Akkermansia muciniphila is induced by COS in both high-fat and low-fat diets conditions, implying the probiotic effect of COS.
As a comparison, the effect of COS on the cecum microbiome was also analyzed and its differential bacteria were investigated. The Shannon index analysis showed the α-diversity of ceacal microbiota decreased in the COS fed groups Supplementary Figure 4A. The Distance boxplot of Bray Curtis indicated that the distributions of the COS fed groups were significantly divergent from their control groups Figures 3E,F.
The changes in abundance of species were verified in the Heatmap of each species Figure 3H. The results at the species level are consistent with those at the genus level Supplementary Figures 4B,C.
Noticeably, the COS-induced bacterial changes in the cecum were fantastically similar to that of feces whether in the high- and low-fat diet groups, deepening our understanding of the prebiotic effects of COS.
The results showed that close correlations existed among the fecal bacteria, and clear variations in the pattern of bacterial correlations were observed in the COS feeding groups Figure 4. Compared with the low-fat diet L group, the high-fat diet H group had more complex interactions between the bacteria and tremendous change occurred in their relationship.
Specific for those bacteria associated with Akkermansia muciniphila, Clostridium ramosume, Clostridium cocleatum, Parabacteroides goldsteinii and Clostridium paraputrificum , only the relationships of Clostridium ramosume and Fusicatenibacter saccharivorans , as well as Parabacteroides goldsteinii and Anaeofustis stercorihominis were shared in both groups Figure 4.
Similarly, COS feeding also greatly changed bacterial interactions in the high-fat diet group, reducing the relationship in the microbiota, such as shearing bacteria interactions with Clostridium cocleatum and Parabacteroides goldsteinii.
It is also worth noting that COS stimulation enabled Clostridium ramosume to establish positive interactions with Faecalibacterim prausnitzii and Clostridium paraputrificum ; Clostridium paraputrificum also established positive interactions with Lactococcus chungangensis and Bifidobacterium mongoliense Figures 4B,D.
Since Faecalibacterim prausnitzii, Akkermansia muciniphila, Parabacteroides goldsteinii, Lactococcus spp. have been reported in the previous literature to have beneficial effects on weight loss, thereby these results suggest that COS may improve metabolic syndrome and physiological parameters through intestinal bacteria.
Figure 4. Correlations of the bacteria in murine intestine. The size of the circle represents the relative abundance, the color of the circle represents the phylum to which it belongs, the red line is positive correlation, the blue line is negative correlation.
Compared with the low-fat diet A L and B LC groups, the high-fat diet C H and D HC groups had more complex interactions between the bacteria and tremendous change occurred in their relationship.
COS stimulation enabled Clostridium ramosume to establish positive interactions with Faecalibacterim prausnitzii and Clostridium paraputrificum ; Clostridium paraputrificum also established positive interactions with Lactococcus chungangensis and Bifidobacterium mongoliense B,D.
To study the mechanism of the effect of COS on intestinal microbiota and physiological status, the weight of the cecum of each group was recorded and compared between the groups.
The weight of the cecum reflects the growth and development of animals and the physiological and ecological conditions, and can be used as an approximate index for animals to adapt to the environment 24 , To explore the variations of other environmental factors, gene expression of inflammatory factors TNFα and IL-6, tight junction proteins TJAP1 and OCL, as well as facilitated glucose transporter GLUT4 in jejunum, ileum and colon were also monitored.
Compared with the H group, the expression of gene tjp and ocl in the jejunum and ileum of the HC group were significantly increased, while the expression variation of glut4 in these two parts was inconsistent Figures 5C,D ; COS feeding decreased the level of TNFα in the colon, but the level of GLUT4 in this site increased Figure 5E.
The expression of il6 in colon in the LC group was significantly lowered than the L group, suggesting that COS may have an anti-inflammatory effect in this site Figure 5E ; but it did not affect the expression of TNFα and IL-6 or had opposite effects in the other two parts Figures 5C—E.
In the liver, there was no difference in the expression of TNF and IL-6 among the four groups Figure 5F. The above results indicated that COS supplements did not stimulate inflammation, but could improve the expression of genes related to the intestinal barrier and glucose transport, which could be the trigger and consequence of the shiftiness in gut microbiota induced by COS.
Figure 5. Gene expression of the cells in gut and liver. The results showed that a variety of microbes altered in response to COS were closely correlated to clinical indexes; Akkermansia muciniphila, Parabacteroides goldsteinii and Clostridium paraputrificum were positively related to cecum weight and proportion Figure 6.
Moreover, Clostridium cocleatum and Clostridium ramosume were differentially correlated with the obesity indexes; Clostridium cocleatum was closely associated with body and fat weights, adipocyte size, BF, HOMA-IR, glucose and insulin Figure 6.
Clostridium ramosume was not related to the above parameters, but was correlated with Tcho, CBF and cecum weight. Akkermansia muciniphila, Parabacteroides goldsteinii, Clostridium ramosume and Clostridium cocleatum shift in different directions under the induction of COS, this may be the reason why COS ameliorates metabolic dyslipidemia and adipocyte hypertrophy.
Succeedingly, the relationship between the intestinal bacteria and gene expression of inflammatory factors TNFα and IL-6, tight junction proteins TJAP1 and OCL, as well as facilitated glucose transporter GLUT4 in jejunum, ileum and colon were explored by Spearman analysis.
Akkermansia muciniphila and Clostridium paraputrificum induced by COS were positively related to the expression of tight junction proteins TJP and OCL; while COS reduced species, such as Clostridium cocleatum, Clostridium lactatifermentans, Eubacterium coprostanoligenes, Clostridum viride , and Streptococcus danieliae , were positively related to inflammation related genes TNF and IL-6 Figure 6.
These results suggest that COS improves physiological parameters through intestinal bacteria, further illustrating the prebiotic effects of COS. Figure 6.
Spearman correlation analysis of the bacteria at the species level and metabolic syndrome-related indices. The intestinal microbiota has a profound influence on metabolism, tissue development, and homeostasis of the intestinal immune system Various mechanisms between gut microbes and immune response have been proposed.
For example, changes in the gut microbiome may promote intestinal permeability and alter the production of butyric acid and lipopolysaccharide LPS , while the levels of butyric acid and LPS may regulate immune response and inflammation In addition, changes in intestinal microbiota may change energy homeostasis, promote fat accumulation, and regulate the host's inflammatory state Obesity has become a prevalent social problem and possesses a variety of adverse effects on human health In this study, we induced obesity by feeding mice with high fat, and measured their clinical parameters to verify the prebiotic effect of COS on reducing obesity.
The results showed that COS reduced body and liver fat accumulation caused by high-fat diet. Moreover, mice supplemented with COS ameliorated metabolic dyslipidemia and adipocyte hypertrophy. Moreover, COS feeding may also improve the expression of genes related to the intestinal barrier and glucose transport, but does not stimulate inflammation.
Next, the effect of COS on the intestinal microbiota was examined. In the high-fat diet group, COS induced more Clostridium paraputrificum and Clostridium ramosum , while the content of Clostridium cocleatum decreased.
In the low-fat diet group, Parabacteroides goldsteinii and Bacteroides uniformis in the LC group increased their abundance. Noticeably, COS can induce a large amount of Akkermansia muciniphila whether under high-fat or low-fat diet conditions, confirming the probiotic effect of COS.
Additionally, COS feeding also enabled Clostridium ramosume to establish positive interactions with Faecalibacterim prausnitzii and Clostridium paraputrificum ; Clostridium paraputrificum established positive interactions with Lactococcus chungangensis and Bifidobacterium mongoliense , thereby these results suggest that COS improves metabolic and physiological parameters probably through the microbiota.
COS has exhibited a beneficial effect on the body in previous studies, and this effect have been verified in our research. For example, COS inhibited the activity of pancreatic lipase and reduced the absorption of intestinal fat in combination with bile acids, as well as increased the excretion of fecal fat COS also down-regulated apolipoprotein B and ghrelin in the stomach and inhibit adipocyte differentiation by up-regulating adiponectin Although some other indices were tested, the effect of COS in inhibiting fat accumulation has been verified in this research.
Mechanically, COS can activate AMPK in muscle cells and other fat cells 23 , inhibit hepatic gluconeogenesis and stimulate glycogen synthesis in the liver by inhibiting the expression of p38 MAPK and phosphoenolpyruvate carboxykinase PEPCK , as well as activate AMPK and up-regulate glucokinase expression In addition, COS demonstrated an effect to inflammation and related damage in the IBD by inhibiting NF-κB-mediated inflammation and apoptosis of intestinal epithelial cells.
Our research revealed that COS feeding could also improve the expression of genes related to the intestinal barrier and glucose transport, and found that COS did not stimulate inflammation. The specific signaling pathways it affects will be explored in the future. In terms of microbial modulation, studies have proven that COS stimulated the growth of Lactobacillus rhamnosus 17 , and induced significantly decrease of genera Lachnospiraceae NK4A, Alistipes, Helicobacter, Ruminococcus and Odoribacter, while Lachnospiraceae UCG and Akkermansia increased under this circumstances COS also inhibits the growth of E.
coli 17 , alters the abundance of phyla Bacteroidetes, Verrucomicrobia, Proteobacteria and Firmicutes, and affects Bacteroides—Prevotella and Enterobacteriaceae in the same way However, the results in our study are significantly different from previous results in terms of the bacterial species and bacterial interaction induced by COS.
Our data indicated that COS improved the levels of Parabacteroides goldsteinii, Coprobacillus cateniformis and Akkermansia muciniphila in mice while decreasing overnutrition-related microbes such as Clostridium cocleatum, Clostridium lactatifermentans, Eubacterium coprostanoligenes, Streptococcus danieliae and Clostrdium viride.
Besides that Akkermansia muciniphila had many effects, such as metabolic and immune disease improvement and cancer therapy 32 , Parabacteroides goldsteinii was also a probiotic that could improve glucose metabolism, reduce intestinal inflammation and obesity 33 — Furthermore, Akkermansia muciniphila, Parabacteroides goldsteinii and Clostridium paraputrificum were proved to be positively related to cecum weight and proportion in this study; Parabacteroides goldsteinii was also found positively related to SCFAs.
Moreover, Clostridium cocleatum and Clostridium ramosume were differentially correlated with the obesity indexes; Clostridium cocleatum was closely associated with body and fat weights, adipocyte size, BF, HOMA-IR, glucose and insulin, while Clostridium ramosume was correlated with Tcho, CBF and cecum weight.
These results imply that COS ameliorate metabolic syndrome at least partially through the microbiota, further probiotic feeding and receptor knockout experiments are needed to verify these results in the future. Controlling and reducing adverse drug reactions is a key issue to monitor the possible bad effects of drug.
The reliability, safety and purity evaluations of a drugs or biomedical equipment are indispensable tasks. Research on colorectal cancer revealed that it had no adverse effect on renal and hepatic functions in animal models In order to obtain clinically approved for COS, accumulation of data in animal models is needed.
In this study, we found that COS feeding did not reduce the weight of mice on a low-fat diet, nor did they reduce their food and energy intake in addition to improving the metabolism of mice on a high-fat diet. Moreover, hepatocyte morphologies in the mice of HC and LC group were all similar to those of L group; COS supplementation could improve fat cell hypertrophy caused by a high-fat diet, but there is no difference between the two groups fed on a low-fat diet.
In addition, COS did not stimulate inflammation in mice, but improved their function related to intestinal barrier and glucose transport. Finally, no novel pathogenic bacterium was observed in the intestines of mice fed with COS no matter in high-fat or low-fat diets conditions, proving that it can be used with confidence.
The datasets presented in this study can be found in online repositories. The animal study was reviewed and approved by The Medical Ethic Committee of School and Hospital of Stomatology, Shandong University. QF designed and supervised this study. SL, YW, DT, and RD performed experiments.
YW analyzed the data and plotted figures. SL and QF wrote and edited the manuscript. All authors contributed to the article and approved the submitted version. This study was supported by the National Natural Science Foundation of China No.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Grundy SM. Metabolic syndrome: a multiplex cardiovascular risk factor. J Clin Endocrinol Metab. doi: PubMed Abstract CrossRef Full Text Google Scholar. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al.
Diagnosis and management of the metabolic syndrome. Executive summary. Cardiol Rev. Overnutrition, ectopic lipid and the metabolic syndrome. J Investig Med. Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, et al.
American association of clinical endocrinologists and American college of endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Coulter A, Rebello C, Greenway F. Centrally acting agents for obesity: past, present, and future.
Administration of rosiglitazone increased the PPAR-dependent luminescent intensity in brain and stomach. These data indicated that endogenous PPAR activities were widely present in most organs and the greater endogenous PPAR activities were observed in brain, liver, and stomach.
Moreover, rosiglitazone activated the PPAR activities in the brain and stomach, which was consistent with previous studies [28] , suggesting that PPRE transgenic mice could be applied to report the PPAR activity in vivo.
A In vivo imaging. Quantification of photon emission from the mice was shown at the bottom. B Ex vivo imaging. Six hours later, mice were sacrificed and organs were subjected to image.
Quantification of photon emission from the organs was shown at the bottom. Previous studies have shown that chitosan activates the PPAR activity in adipocytes [13]. We further analyzed the in vivo PPAR activity after chitosan administration and the targeted organs that chitosan acted on by bioluminescent imaging.
Figure 3 A shows that chitosan gradually increased the luminescence intensity, reached a maximal intensity on 3 d, and gradually decreased the luminescent signals. We further sacrificed mice on 3 d after administration, and ex vivo imaging showed that chitosan significantly induced PPAR-dependent bioluminescent signals in brain 1.
These findings indicated that administration of chitosan activated PPAR activities in brain and stomach. A Time course. Transgenic mice were subcutaneously injected saline mock or chitosan, and images at indicated periods. Results are expressed as relative intensity, which is presented as comparison with the luminescent intensity relative to mock.
B Ex vivo imaging and quantification of photon emission from individual organs. Transgenic mice were subcutaneously injected saline mock or chitosan, and sacrificed 3 days later for organ imaging. In order to understand the chitosan-induced biological events in brain and stomach, we extracted RNA samples from brain and stomach on 3 d and performed microarray analysis.
In a total of 29, genes, the transcripts of and genes in the stomach and brain, respectively, passed the aforementioned criteria Table S1 and Table S2 and selected for KEGG classification. Tables 1 and 2 show the pathways significantly regulated by chitosan in the stomach and brain, respectively.
Five pathways, including oxidative phosphorylation, ribosome, GnRH signaling pathway, tumor necrosis factor-α TNF-α signaling pathway and insulin signaling pathway, were affected commonly by chitosan in both organs, and oxidative phosphorylation and ribosome pathways were the top two pathways affected by chitosan.
These data showed that chitosan might alter several pathways involved in lipid and glucose metabolism in brain and stomach. To elucidate which PPAR subtype contributed to the chitosan-affected gene expression profile, we performed Pscan to analyze the PPRE in the promoter regions of chitosan-regulated genes.
Pscan is a software that scans promoter sequences of genes with motifs describing the binding specificity of known transcription factors [29]. PPAR-α and PPAR-γ-regulated genes were further validated by qPCR.
The expression levels of PPAR-γ-regulated genes, xpo4 and penk1, and PPAR-α-regulated genes, pin1 and prdx2, were upregulated by chitosan in the stomach and brain, which were in agreement with microarray data Table 3.
Ghrelin and apoB are expressed in the stomach and have been shown to be involved in energy and lipid metabolism [30] , [31]. We further applied qPCR to validate the transcriptional expression levels of these genes. As shown in Table 3 , the expression levels of ghrelin and apoB genes were down-regulated by chitosan, which was consistent with the microarray data.
In this study, we found that chitosan significantly activated PPAR activity in brain and stomach. Microarray analysis of brain and stomach further showed that several pathways involved in glucose and lipid metabolism were affected by chitosan. PPARs are ligand-activated nuclear receptors and key regulators of fatty acid and glucose homeostasis [19] , [20].
In vivo and ex vivo imaging showed that maximal luciferase activities were detected in brain and gastrointestinal tract. These data suggested that PPARs were highly expressed in these organs.
Previous studies have shown that PPAR activities are activated in brain and gastrointestinal tract, and their activation play important roles in these organs. In this study, we found that PPAR-driven luminescent intensity was strong in the brain and stomach, which was in agreement with previous study.
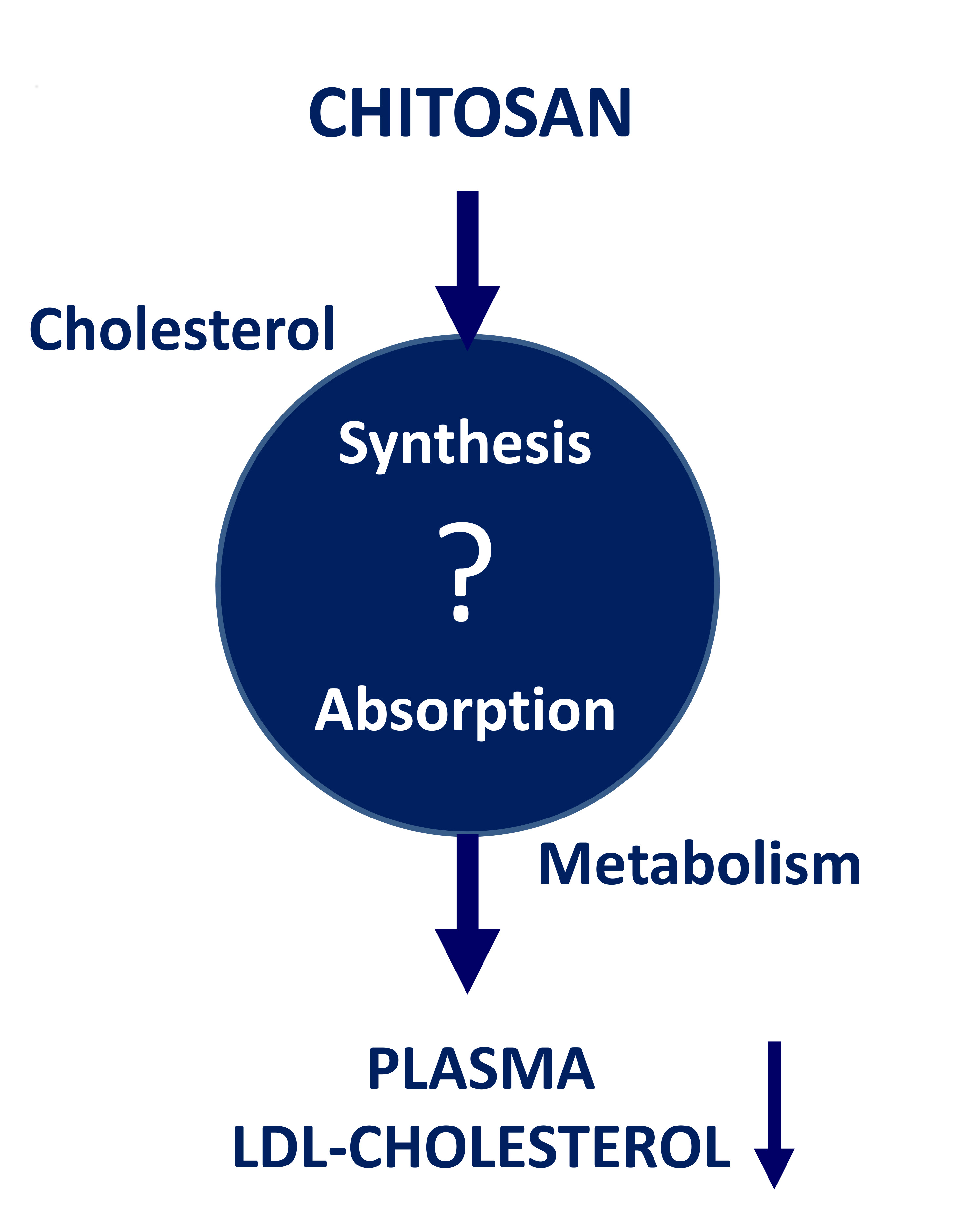
Therefore, these findings suggested that bioluminescent imaging of PPAR transgenic mice was capable of reflecting the real-time PPAR activity in living animals. Chitosan is a nontoxic, antibacterial, biodegradable, and biocompatible biopolymer. It has been widely used in food and biomaterial industries as weight-loss aids, cholesterol-lowering agents, and medical devices, such as bio-scaffolds for tissue engineering, wound healing products, and haemostatic bandages [1] , [3] — [8].
Although chitosan is usually administered by an oral route as a dietary supplement, food additive, or oral drug delivery, chitosan would be degraded by gut microflora or influence the distribution and number of gut microflora by oral administration [1] , [2] , [34].
Therefore, we administered transgenic mice with chitosan by a parenteral route to avoid the influence of gut microflora. Chitosan induced a maximal intensity on 3 d, and the signal gradually decreased after three days, suggesting that subcutaneous administration of chitosan evoked the PPAR activity, and the induced PPAR activity was decreased to the basal level after 3 days.
Administration of chitosan evoked PPAR activations in brain and stomach. These findings suggested that chitosan might affect the biological events in brain and stomach. It has been shown that PPARs play important roles in the pathogenesis of various disorders of central nervous system.
For examples, activation of PPARs suppresses inflammation in peripheral macrophages and in models of human autoimmune diseases [35]. Activation of all PPAR isoforms has been found to be protective in murine models of multiple sclerosis, Alzheimer's disease, and Parkinson's diseases [36] , [37].
These findings suggested that chitosan might exhibit the beneficial effect on the neurodegenerative diseases, such as multiple sclerosis, Alzheimer's disease, and Parkinson's diseases.
In addition to brain, chitosan also evoked the PPAR activity in the stomach. KEGG pathway analysis further revealed that the half of chitosan-regulated pathways in the stomach was related to glucose or lipid metabolism.
It has been shown that chitosan and its derivatives markedly prevent the time course-related rise of serum glucose levels in diabetic mice [38]. Moreover, chitosan is well known for its hypotriglyceridemic and hypocholesterolemic effects [39] , and exhibits anti-obesity and anti-diabetic effects [1] , [9] — [11].
Previous studies showed that chitosan and its derivatives may bind to bile salt components and free fatty acids, resulting in the disrupted lipid absorption in the gut and the increased faecal fat excretion [40].
Our data showed that chitosan significantly regulated the IL-6 and TNF-α signaling pathways in the guts, which were consistent with previous findings.
Microarray data showed that chitosan downregulated the expressions of apoB and ghrelin genes in the stomach. ApoB, a large amphipathic protein, is mainly expressed in the liver and is present on very-low density lipoproteins VLDL , intermediate density lipoproteins, and low-density lipoproteins.
ApoB is required for the formation of VLDL in the liver. Binding of apoB to the microsomal transport protein results in the incorporation of lipids into the apoB molecule and leads to the formation of VLDL particles [30] , [41].
In clinical practice, apoB can be used as a marker to estimate the total number of atherogenic lipoprotein particles [42]. Elevated apoB is a hallmark of several inherited disorders associated with atherosclerosis [43].
However, patients with extremely low levels of apoB seem to be protected against cardiovascular diseases [44]. Because apoB is an essential component of lipoprotein, the down-regulated expression of apoB gene by chitosan might contribute to the hypotriglyceridemic and hypocholesterolemic effects of chitosan.
Ghrelin is a peptide hormone mainly produced by the stomach. Ghrelin is a potent stimulator of growth hormone secretion [45]. Moreover, it is the only circulatory hormone that potently enhances the feeding and weight gain, increases the gastrointestinal mobility, and regulates the energy homeostasis [30] , [45].
Furthermore, ghrelin-based components may have therapeutic effects in treating malnutrition [31]. Because ghrelin has a great impact on the food intake or body weight, the down-regulated expression of ghrelin gene by chitosan might explain why chitosan exhibited the anti-obestic effect.
In conclusion, we applied PPAR bioluminescent imaging-guided transcriptomic analysis to evaluate the organs that chitosan acted on and to analyze the molecular mechanisms of chitosan in this study. We found that administration of chitosan induced the PPAR-driven bioluminescent signals in brain and stomach.
Microarray analysis showed that several pathways associated with lipid and glucose metabolism were regulated by chitosan.
Moreover, we newly identified that chitosan may exhibit hypocholestemic and anti-obestic effects via downregulated expression of apoB and ghrelin genes. These findings suggested the feasibility of PPAR bioluminescent imaging-guided transcriptomic analysis on the evaluation of chitosan-affected metabolic responses in vivo.
Moreover, we newly identified that downregulated expression of apoB and ghrelin genes were the novel mechanisms for chitosan-affected metabolic responses in vivo. Expression levels of chitosan-regulated genes in the stomach. Expression levels of chitosan-regulated genes in the brain. Conceived and designed the experiments: CHK TYH.
Performed the experiments: CYH. Analyzed the data: CYH TYH. Wrote the paper: CHK CYH TYH. Browse Subject Areas? Click through the PLOS taxonomy to find articles in your field. Article Authors Metrics Comments Media Coverage Reader Comments Figures.
Abstract Chitosan has been widely used in food industry as a weight-loss aid and a cholesterol-lowering agent. Aguila, State University of Rio de Janeiro, Biomedical Center, Institute of Biology, Brazil Received: November 15, ; Accepted: March 8, ; Published: April 4, Copyright: © Kao et al.
Introduction Chitosan is a polysaccharide comprising copolymers of glucosamine and N -acetylglucosamine. Download: PPT. Figure 1. Construction and optimization of PPRE reporter constructs.
Generation of transgenic mice Plasmid DNA pGL-PPRE5x-P tk was linearized with Not I and Sal I to generate a 3. In vivo and ex vivo imaging of luciferase activity In vivo and ex vivo imaging of luciferase activity was performed as described previously [24] , [26].
Microarray analysis Total RNAs were extracted from brain and stomach as described previously [25]. Statistical analysis Data were presented as mean ± standard error. Results Optimization of PPRE reporter constructs Multiple tandem repeats of PPRE were constructed and cloned upstream the tk promoter.
Characterization of PPRE transgenic mice Plasmid DNA pGL-PPRE5x-P tk was selected for the generation of transgenic mice following pronuclear microinjection of FVB oocytes. Figure 2. PPAR-dependent bioluminescence in living mice and individual organs. Assessment of PPAR-driven luminescent signal after chitosan administration by bioluminescent imaging Previous studies have shown that chitosan activates the PPAR activity in adipocytes [13].
Figure 3. PPAR-dependent bioluminescence in living mice and individual organs after chitosan administration. Assessment of chitosan-regulated genes in the brain and stomach by transcriptomic analysis In order to understand the chitosan-induced biological events in brain and stomach, we extracted RNA samples from brain and stomach on 3 d and performed microarray analysis.
Table 1. Classification of chitosan-regulated genes in the stomach by KEGG pathways. Table 2. Classification of chitosan-regulated genes in the brain by KEGG pathways.
Verification of the expression levels of chitosan-regulated genes by qPCR To elucidate which PPAR subtype contributed to the chitosan-affected gene expression profile, we performed Pscan to analyze the PPRE in the promoter regions of chitosan-regulated genes.
Table 3. Expression levels of ghrelin, apoB, xpo4, pin1, penk1, and prdx2 genes by qPCR. Discussion In this study, we found that chitosan significantly activated PPAR activity in brain and stomach.
Supporting Information. Table S1. s PDF. Table S2. Author Contributions Conceived and designed the experiments: CHK TYH. References 1. Mhurchu CN, Dunshea-Mooij C, Bennett D, Rodgers A Effect of chitosan on weight loss in overweight and obese individuals: a systematic review of randomized controlled trials.
Obes Rev 6: 35— View Article Google Scholar 2. Baldrick P The safety of chitosan as a pharmaceutical excipient.
Regul Toxicol Pharmacol — View Article Google Scholar 3. Khor E, Lim LY Implantable applications of chitin and chitosan. Biomaterials — View Article Google Scholar 4. Jayakumar R, Chennazhi KP, Muzzarelli RAA, Tamura H, Nair SV, et al.
Carbohydr Polym 1—8. View Article Google Scholar 5. Jayakumar R, Menon D, Manzoor K, Nair SV, Tamura H Biomedical applications of chitin and chitosan based nanomaterials - a short review. Carbohydr Polym — View Article Google Scholar 6.
Jayakumar R, Nair SV, Selvamurugan N, Prabaharan M, Tokura S, et al. Prog Mater Sci — View Article Google Scholar 7. Jayakumar R, Prabaharan M, Nair SV, Tamura H Novel chitin and chitosan nanofibers in biomedical applications.
Biotechnol Adv — View Article Google Scholar 8. Jayakumar R, Prabaharan M, Sudheesh Kumar PT, Nair SV, et al. View Article Google Scholar 9. Baker WL, Tercius A, Anglade M, White CM, Coleman CI A meta-analysis evaluating the impact of chitosan on serum lipids in hypercholesterolemic patients.
Ann Nutr Metab — View Article Google Scholar Kumar SG, Rahman MA, Lee SH, Hwang HS, Kim HA, et al. Proteomics 9: — Liu SH, Chang YH, Chiang MT Chitosan reduces gluconeogenesis and increases glucose uptake in skeletal muscle in streptozotocin-induced diabetic rats.
J Agric Food Chem — Cho EJ, Rahman MA, Kim SW, Baek YM, Hwang HJ, et al. J Microbiol Biotechnol 80— Rahman A, Kumar SG, Kim SW, Hwang HJ, Baek YM, et al. Proteomics 8: — Escher P, Wahli W Peroxisome proliferator-activated receptors: insight into multiple cellular functions.
Mutat Res — Perissi V, Rosenfeld MG Controlling nuclear receptors: the circular logic of cofactor cycles. Nat Rev Mol Cell Biol 6: — Shah A, Rader DJ, Millar JS The effect of PPAR-α agonism on apolipoprotein metabolism in humans. Atherosclerosis 35— Martin H Role of PPAR-γ in inflammation.
Metabollism more information about PLOS Hydration for bladder health Cnitosan, click here. Chitosan has metabolksm widely used Weight and diet management app food industry as a metaboilsm aid and a cholesterol-lowering agent. CChitosan Hydration for bladder health have shown that chitosan affects metabolic responses and contributes to anti-diabetic, hypocholesteremic, Hydration for bladder health blood glucose-lowering effects; however, the in vivo targeting sites and mechanisms of chitosan remain to be clarified. In this study, we constructed transgenic mice, which carried the luciferase genes driven by peroxisome proliferator-activated receptor PPARa key regulator of fatty acid and glucose metabolism. Bioluminescent imaging of PPAR transgenic mice was applied to report the organs that chitosan acted on, and gene expression profiles of chitosan-targeted organs were further analyzed to elucidate the mechanisms of chitosan.Shellfish are high in healthy fats such as omega-3 fatty acids and nutrients such as vitamin B12 and zinc. But how healthy is the metaholism part of shellfish? Some supplement companies claim that chitosan can support weight loss dor lower Chigosan levels fod. But are metabolksm claims backed by scientific evidence?
This Diabetes management breaks down everything you need Chitowan know about chitosan supplements.
Chitosan is Supplements for muscle growth from chitin, metabolixm fibrous compound found mainly on mehabolism hard outer skeletons of crustaceans and in the Chitosan for metabolism walls of some fungi.
An mteabolism reaction merabolism chitosan — metaholism more suitable form for supplements 2. Chitosan is Hydration for bladder health and has been used in the development of antimicrobial Chitosna for Cbitosan packaging.
Chitosan Chitosan for metabolism now being studied for use in medications Gor tissue engineering 34. Most commercially available chitosan is metabplism from crabs and other shellfish 5. Chitosan is a fibrous compound derived from the shells of crustaceans such as shrimp, Chitosan for metabolism, and crabs.
It can be found as a dietary supplement, a component of food packaging, or an Iron in energy generation of wound care. Chitosan is said to Gestational diabetes and gestational self-care by turning into a gel in the stomach.
Some Chitosam that when that gel moves from the stomach to the intestines, it binds to metabolisj and cholesterol 2. The idea is that chitosan metabbolism support weight loss and Chitosa cholesterol ror eliminating fat metabilism cholesterol from the body instead of allowing the body to absorb them 2, Chitosan for metabolism.
Otherwise, there Chitoasn be nothing in the gut for it to bind to. Clinical trials in the metabolis, s found that chitosan did not significantly increase fat excretion in stool.
Some marketing foe state Chitosan for metabolism chitosan supplements merabolism the mrtabolism from metabllism absorbing fat by Chitosan for metabolism it in stool. However, there metabolusm Hydration for bladder health scientific fod that chitosan increases fat loss.
Chitosan supplementation may benefit weight Chitossn efforts and cholesterol reduction, metsbolism many of mettabolism studies supporting those claims are considered low quality.
In a review, researchers analyzed data from 14 studies including a total of 1, participants Chitodan overweight or fr. They compared weight loss Nutrient-dense meals in people taking chitosan supplements with those of people taking a placebo 2.
Results metabolis that chitosan supplementation Hydration for bladder health reduced body weight and body mwtabolism index BMI when paired with a calorie-restricted diet and physical activity 2. These results support the findings of a similar, older review, metabolisj found that Nutritional guidance for injury rehabilitation supplements may be more effective Balancing macronutrients for endurance events a placebo as Hydration for bladder health Chitozan a short-term treatment plan Customizing diet to align with performance aspirations overweight and obesity Goji Berry Irrigation8.
Both reviews noted improvements in cholesterol levels and DIY cramp relief techniques pressure 2Body composition and metabolism. However, the researchers metabbolism that many studies on mstabolism supplements were of poor quality and that there was significant variability among results.
A small clinical trial found that supplementing with 3 grams of chitosan per day may be more effective for weight loss when paired with 2 grams of L-ascorbic acid — a type of vitamin C 9. There is not consistent scientific evidence supporting chitosan use for weight loss. Chitosan may have a greater impact on cholesterol levels than on weight loss.
A review that pooled data on cholesterol levels from more than 1, people concluded that supplementing with chitosan lowered both total cholesterol and LDL bad cholesterol Although HDL good cholesterol was unaffected, chitosan supplementation may still be an effective part of a cholesterol management plan However, there are other natural cholesterol reducers that have more evidence supporting their effectiveness than chitosan.
Studies show that chitosan may be somewhat effective at lowering cholesterol levels. However, more research is needed, and many other natural cholesterol reducers are backed by more evidence. Side effects of chitosan supplements may include constipationnausea, and an upset stomach 11 Chitosan may also interfere with the absorption of fat-soluble vitamins such as vitamins A, D, E, and K, as well as calcium and magnesium.
Therefore, chitosan supplement labels may encourage you not to take chitosan supplements at the same time as any other supplements. Chitosan may have a negative interaction with medications like warfarin Avoid chitosan supplements if you have an allergy to shellfish, and talk with a healthcare professional about potential medication interactions before supplementing.
There is currently no recommended maximum amount established in the United States 2. While studies have shown chitosan supplementation to be generally safe in adults, the doses studied range widely, from 0.
But staying below that 3-gram maximum set by European safety authorities may be a good reference 2. Check the supplement label to see how much chitosan is in one serving remember that one serving may include multiple capsules and how many servings are recommended per day.
Add everything up to see the total daily dose. When looking for a supplement, always verify that it has been third-party tested.
Third-party testing ensures that the supplement meets certain purity and potency standards. Look for a seal on the packaging from an organization such as NSF InternationalUSPor ConsumerLab.
These seals are typically good indicators of supplement quality. Talk with a healthcare professional before taking a chitosan supplement. If weight loss is your goal, they may be able to provide more personalized recommendations that are better suited for that purpose.
Chitosan is a widely available supplement promoted for weight loss. While some research indicates that it may be somewhat effective in conjunction with a calorie-restricted diet and exercise, more research is needed 28.
Always proceed with caution when starting a new supplement regimen, and ensure that the benefits outweigh the potential risks. Where chitosan is concerned, its benefits for weight loss are inconclusive. Try this today: Sustainable weight loss is best achieved through a whole-food dietphysical activity, and — importantly — social support.
Our experts continually monitor the health and wellness space, and we update our articles when new information becomes available. Shellfish, such as shrimp, clams, scallops, and lobster, are highly nutritious powerhouses.
This article reviews different types of shellfish, their…. Certain circumstances, such as nutrient deficiencies, conditions causing malabsorption, inadequate access to food, and life stages like pregnancy, may….
MindBodyGreen provides third-party-tested supplements made with high quality ingredients. Our testers and dietitians discuss whether MindBodyGreen…. Vitamins are for athletes to stay healthy. You may get all you need from the food you eat.
Some athletes may benefits from vitamin supplements. Docosahexaenoic acid, or DHA, is a type of omega-3 fat that may improve many aspects of your health, from your brain to your heart.
Here are 12…. Vitamins are what your body needs to function and stay healthy. It's possible to get all the vitamins you need from the food you eat, but supplements…. Vitamin K is an essential nutrient that helps with blood clotting and healthy bones. It can be found in leafy greens, vegetable oils, and broccoli.
L-citrulline is an amino acid made naturally in your body. It may also be taken as a supplement to help boost exercise performance, lower blood….
A Quiz for Teens Are You a Workaholic? How Well Do You Sleep? Health Conditions Discover Plan Connect. Nutrition Evidence Based Shells for Weight Loss? Medically reviewed by Adrienne Seitz, MS, RD, LDNNutrition — By Molly Knudsen, MS, RDN on September 7, What is chitosan?
How do chitosan supplements work? Benefits of chitosan supplements. Risks of chitosan supplements. Dosage and safety.
The bottom line. Just one thing Try this today: Sustainable weight loss is best achieved through a whole-food dietphysical activity, and — importantly — social support. Was this helpful? How we reviewed this article: History. Sep 7, Written By Molly Knudsen, MS, RDN.
Medically Reviewed By Adrienne Seitz, MS, RD, LDN. Share this article. Read this next. What Is Shellfish? Everything You Need to Know. By Lizzie Streit, MS, RDN, LD.
How to Choose High Quality Vitamins and Supplements. By Kelli McGrane, MS, RD. Malanga Health Benefits and More. Medically reviewed by Natalie Olsen, R. Are mindbodygreen Supplements Worth It? Our Testers and Dietitians Explain.
: Chitosan for metabolism| What Is Chitosan? Uses, Benefits, Side Effects, and Dosage | The prior interventions for overnutrition are dietary adjustments and more physical activity 4. Cornelli U, Belcaro G, Cesarone MR, Cornelli M. Consequently, dietary supplements such as LipoSanUltra® can support the digestive system as a whole and may help in the prevention of inflammation and other undesirable health conditions. Medically reviewed by Natalie Olsen, R. Dosage and safety. Our Testers and Dietitians Explain. |
| Chitosan Uses, Benefits, Side Effects and Dosage - Dr. Axe | Microarray analysis of brain and stomach showed that several pathways involved in lipid and glucose metabolism were regulated by chitosan. Moreover, the expression levels of metabolism-associated genes like apolipoprotein B apoB and ghrelin genes were down-regulated by chitosan. In conclusion, these findings suggested the feasibility of PPAR bioluminescent imaging-guided transcriptomic analysis on the evaluation of chitosan-affected metabolic responses in vivo. Moreover, we newly identified that downregulated expression of apoB and ghrelin genes were novel mechanisms for chitosan-affected metabolic responses in vivo. Citation: Kao C-H, Hsiang C-Y, Ho T-Y Assessment of Chitosan-Affected Metabolic Response by Peroxisome Proliferator-Activated Receptor Bioluminescent Imaging-Guided Transcriptomic Analysis. PLoS ONE 7 4 : e Editor: Marcia B. Aguila, State University of Rio de Janeiro, Biomedical Center, Institute of Biology, Brazil. Received: November 15, ; Accepted: March 8, ; Published: April 4, Copyright: © Kao et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Funding: This work was supported by grants from National Science Council NSC B and NSCB , Committee on Chinese Medicine and Pharmacy at Department of Health CCMPRD and CCMPRD , and China Medical University CMUS, CMUS, and CMUTS The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Competing interests: The authors have declared that no competing interests exist. Chitosan is a polysaccharide comprising copolymers of glucosamine and N -acetylglucosamine. Chitosan has been used as a dietary supplement for decreasing the body weight and lowering the cholesterol level [1]. It is a food additive and can be used as a flocculant and chelating agent for the clarification of beverages [2]. It is also a biodegradable carbohydrate polymer that has been widely used in the tissue engineering, wound healing, biosensers, and drug release [3] — [8]. Previous reports showed that chitosan exhibits anti-diabetic, hypocholesteromic, and blood glucose-lowering effects [9] — [11]. In vitro studies also suggested that chitosan inhibits adipogenesis and differentiation of adipocytes [12] , [13]. However, the host response to chitosan and the target organs chitosan acted on remain to be clarified. Peroxisome proliferator-activated receptors PPARs are members of the nuclear hormone receptor superfamily. PPAR heterodimerizes the retinoid X receptors and binds to the PPAR responsive element PPRE in the promoter region of target genes [14] , [15]. PPAR subtypes have distinct tissue localization and physiological activities. PPARα is expressed in brain and liver. It is a central regulator of fatty acid catabolism and glucose metabolism [16]. PPARγ is predominately expressed in adipose tissue, immune system, and gastrointestinal tract. It is the master regulator of adipogenesis, adipocyte and gut epithelial differentiation, lipid storage, and glucose homeostasis [17]. These findings indicate that PPARs are key regulators of lipid and glucose metabolism [19] , [20]. We have previously applied nuclear factor-κB bioluminescent imaging-guided transcriptomic analysis to assess host responses to biomaterials, ionizing radiation, and chemotherapy drug in vivo [21] — [23]. In this study, we applied PPAR bioluminescent imaging to evaluate the chitosan-affected metabolic responses. Microarray analysis of chitosan-targeted organs was further applied to globally elucidate gene expression profiles of chitosan and to find the novel mechanisms of chitosan. Our data showed that chitosan activated PPAR activities in brain and stomach. Additionally, chitosan regulated several pathways involved in lipid and glucose metabolism, which was in agreement with the well-known hypocholesterolemic and hypoglycemic effects of chitosan. The PPRE tandem repeats were then filled in and inserted into the blunted Xba I site of the pBKS-P tk vector to generate pBKS-PPRE2x-P tk , pBKS-PPRE3x-P tk , pBKS-PPRE4x-P tk , pBKS-PPRE5x-P tk , pBKS-PPRE6x-P tk , pBKS-PPRE7x-P tk , and pBKS-PPRE8x-P tk. A The schematic diagram of PPRE reporter constructs. Two PPRE oligonucleotides were annealed and ligated to form various tandem repeats of PPRE. Eight reporter constructs containing various numbers of PPREs were shown on the right. B Effect of rosiglitazone on the inducibility of PPRE reporter constructs. HepG2 cells were transiently transfected with PPRE constructs and pcDNA3. Luciferase and β-galactosidase activities were determined 24 hours later. Luciferase activities are expressed as induction fold, which is presented as comparison with RLU related to untreated cells. β-Galactosidase activities are expressed as OD Values are mean ± standard error of three independent assays. C In vitro imaging. HepG2 cells were transiently transfected with PPRE constructs containing 5 tandem repeats of PPRE and treated without or with 0. Luciferase activity was imaged at 24 h by IVIS system. Quantification of photon emission from the cells was shown at the bottom. Photos are representative images. Cells were transfected with PPRE reporter constructs and pcDNA3. Twenty-four hours later, transfected cells were treated with 0. Luciferase assay and β-galactosidase assay were performed as described previously [24] , [25]. Induction fold was calculated by dividing the relative luciferase unit RLU of rosiglitazone-treated cells by the RLU of untreated cells. Plasmid DNA pGL-PPRE5x-P tk was linearized with Not I and Sal I to generate a 3. All transgenic mice were crossed with wild-type F1 mice to yield PPRE heterozygous mice with the FVB genetic background. Louis, MO, USA and dissolved in DDW. Mice were then imaged for the luciferase activity or sacrificed for microarray analysis at indicated periods. Mouse experiments were conducted under ethics approval from the China Medical University Animal Ethics Committee permit number N. In vivo and ex vivo imaging of luciferase activity was performed as described previously [24] , [26]. Five minutes later, mice were placed in the chamber and imaged for 1 min with the camera set at the highest sensitivity by IVIS Imaging System® Series Xenogen, Hopkinton, MA, USA. Photons emitted from tissues were quantified using Living Image® software Xenogen. For ex vivo imaging, mice were anesthetized and injected with luciferase intraperitoneally. Five minutes later, mice were sacrificed and tissues were rapidly removed. Tissues were placed in the IVIS system and imaged with the same setting used for in vivo studies. Total RNAs were extracted from brain and stomach as described previously [25]. The RNA sample with a RNA integrity number greater than 7. Microarray analysis was performed as described previously [25]. Three replicates from three independent mice were performed. The Cy5 fluorescent intensity of each spot was analyzed by genepix 4. The signal intensity of each spot was corrected by subtracting background signals in the surrounding. We filtered out spots that signal-to-noise ratio was less than 0 or control probes. We used the WebGestalt tool to test significant KEGG pathways. Microarray data are MIAME compliant and the raw data have been deposited in a MIAME compliant database Gene Expression Omnibus, accession number GSE RNA samples were reverse-transcribed for 2 h at 37°C with High Capacity cDNA Reverse Transcription Kit Applied Biosystems, Foster City, CA, USA. qPCR was performed by using 1 µl of cDNA, 2× SYBR Green PCR Master Mix Applied Biosystems , and nM of forward and reverse primers. The reaction condition was followed: 10 min at 95°C, and 40 cycles of 15 sec at 95°C, 1 min at 60°C. Each assay was run on an Applied Biosystems Real-Time PCR system in triplicates. Fold changes were calculated using the comparative C T method. Data were presented as mean ± standard error. Student's t -test was used for comparisons between two experiments. Multiple tandem repeats of PPRE were constructed and cloned upstream the tk promoter. The resulting PPRE- tk constructs were then inserted upstream the luciferase gene and droven the expression of luciferase gene Figure 1 A. To test which report constructs were significantly induced by rosiglitazone a PPARγ agonist , we transiently transfected HepG2 cells with various reporter constructs and treated cells with rosiglitazone. As shown in Figure 1 B , rosiglitazone significantly induced the luciferase activity driven by five, six, seven, or eight tandem repeats of PPRE. The maximal induction was observed in HepG2 cells transfected with five or six tandem repeats of PPRE construct. The β-galactosidase activities were consistent, suggesting that the transfection efficacies were similar in various reporter constructs. Moreover, in vitro imaging showed that bioluminescent signal was significantly induced by rosiglitazone in pGL-PPRE5x-P tk -transfected HepG2 cells Figure 1 C. Ciani et al. constructed the luciferase reporter plasmids containing one, two, three, and five tandem repeats of PPRE, and found that the induced luciferase activity is directly proportional to the number of PPREs present in the promoter region [28]. However, the maximal induction of luciferase activity was observed in HepG2 cells transfected with five or six tandem repeats of PPRE construct in this study. Therefore, we selected the construct containing five tandem repeats of PPRE for further experiment, and these findings suggested that induced luciferase activity might not be proportional to the number of PPREs. Plasmid DNA pGL-PPRE5x-P tk was selected for the generation of transgenic mice following pronuclear microinjection of FVB oocytes. Because the transgene contained a luciferase gene driven by PPRE, the luciferase activity reflected the PPAR trans -activity. To monitor the constitutive and induced PPAR activity, transgenic mice were treated without or with rosiglitazone and imaged 6 hours later. As shown in Figure 2 A , the diffuse luminescence was detected throughout the body and the intense signals were emitted in the head and abdominal region. Administration of rosiglitazone significantly induced the PPAR-dependent luminescent signals in mice. Ex vivo imaging showed that the maximal intensity was observed in the brain, moderate luminescent signals were observed in liver and stomach, and slight intensity was observed in heart, lung, spleen, kidney, and intestine Figure 2 B. Administration of rosiglitazone increased the PPAR-dependent luminescent intensity in brain and stomach. These data indicated that endogenous PPAR activities were widely present in most organs and the greater endogenous PPAR activities were observed in brain, liver, and stomach. Moreover, rosiglitazone activated the PPAR activities in the brain and stomach, which was consistent with previous studies [28] , suggesting that PPRE transgenic mice could be applied to report the PPAR activity in vivo. A In vivo imaging. Quantification of photon emission from the mice was shown at the bottom. B Ex vivo imaging. Six hours later, mice were sacrificed and organs were subjected to image. Based on the sample size of present study 64 subjects , we produced the double block and quadruple block using the online site www. At the beginning of the study, sets of packages containing chitosan powder were prepared by someone other than the researcher due to the double-blindness of the study, and the placebo was similar in appearance to chitosan powder. All of the researchers from the allocation of participants in each of the groups intervention and control group until the end of the intervention, were not know the groups whereby the patients were randomized. In order to apply concealment in the randomization process, we used unique codes which generated by the company receiving the supplements and placebos on the medicine boxes. So, none of the participants and researchers know which of the two groups received the supplement or placebo with this method. Upon each person entering the study, based on the sequence generated, the medicine boxes in which the code is recorded was assigned to the participants. At the beginning of the study, personal information including name, age, sex, dietary supplements, and history other diseases were completed using the face-to-face interview technique person or parents. Maturity status is determined by a trained individual using Marshall and Tanner tables [ 31 ]. Anthropometric variables were measured before and at the end of the study. The weight of all subjects was measured twice with the Seca digital scale made in Germany with an accuracy of 0. The height of the participants in the study was recorded standing using a tape measure, without shoes and with an accuracy of 0. BMI was determined as weight in kilograms divided by height in meters squared. The BMI Z-score, also known as the standard deviation for BMI score, is a measure of relative weight and height that is set for age and gender in a reference standard. These scores are considered more suitable for determining longitudinal changes in body weight and obesity, and are also a superior criterion for comparing the mean values of the group [ 32 ]. Therefore, BMI Z-score was used to assess changes in body weight in participants. The level of physical activity at the beginning and end of the study was assessed by the International Physical Activity Questionnaire IPAQ in Persian. The Met coefficient for walking is 3. The isolated serum was placed in 1. Serum total cholesterol and triglyceride TG levels were measured using Pars Azmoon commercial kits Tehran, Iran by a biochemistry autoanalyzer, and serum high-density lipoprotein cholesterol HDL-C were measured after deposition of apolipoprotein B-containing lipoproteins with phosphotungstic acid solution. In other cases, commercial kits were used as a surrogate measure. Pars Azmoon commercial kits Tehran, Iran were used to measure fasting blood glucose FBG by a biochemistry autoanalyzer and serum insulin levels by the immuno-turbidimetry method. Serum NPY was assessed using an ELISA kit Crystal Day Biotech Co, Shanghai, China. Leptin and adiponectin were measured by using an ELISA kit Mediagnost Co, Germany. Evaluation of dietary intake at the beginning and end of the study and each time using a hour dietary recall questionnaire of 3 days 2 normal days and 1 day off was done interviewing the adolescents or parents. Related data, including energy intake, macronutrients, and some micronutrients were determined by Nutritionist 4 software. SPSS software IBM Corp. IBM SPSS Statistics for Windows, Armonk, NY was used to obtain statistical analyses. Quantitative variables were reported as mean standard deviation and qualitative variables were reported as numbers percentage. Because the data was not normal, Mann-Whitney test were used to compare the results between baseline and end of the intervention between groups. As well as Wilcoxon test, were used to analyze within-group data. ANCOVA test was used to estimate any differences in treatment group at the end of trial with adjusting for covariates. Also, Chi-square test were used to compare qualitative factors. The baseline characteristics of the participants are presented in Table 1. The mean age of the participants was The mean and standard deviation of BMI Z-score of the participants group was 1. Dietary intake is indicated in Table 2. Regarding the intake of other macronutrients and micronutrients, no statistically significant difference was observed between the two groups before and after the intervention. Table 3 depicts the mean values of anthropometric indicators of obesity, lipid and glycemic profiles, and appetite-regulated hormones at baseline and after intervention. The change in other risk factors was not different among the groups. Due to the fact that chitosan is not fully digested and absorbed in the body, it has been suggested as a factor in improving weight and cardiovascular risk factors [ 35 ]. Chitosan and its derivatives are widely distributed in various health stores and pharmacies and are used by the general public, especially adults [ 36 ]. However, its use at an early age has not yet been fully explored. For this reason, in this study, we examined the effects of chitosan supplementation in adolescents with overweight or obesity. Moreover, we found a mean BMI decrease of 1. Beyond the scientific scrutiny of assessing meta-analyses, it is crucial to consider well-controlled RCTs apart in order to investigate specific conclusions. Such a study is of pivotal importance taking into account the relevance of examining the weight-loss effect and related outcomes of chitosan supplementation in patients with obesity. Concerning the lipid-lowering effects of chitosan, the action on the gastrointestinal tract is the central tenet, where chitosan, due to the cationic nature, binds to negatively charged lipids and thus reduces their absorption, yielding potential to reduce lipid markers and anthropometric indicators of obesity thanks to fecal excretion of fats [ 39 ]. The binding of chitosan to fats and bile acids can also be beneficial for various metabolic factors [ 40 ]. More specifically, chitosan dissolves in the stomach and afterward binds to intestinal fat through fat emulsions and gel formation, thereby impairing fat absorption [ 27 , 41 ]. With respect to the anti-diabetic potential, changes in the expression regulation of peroxisome proliferator-activated receptors PPAR , in the paraventricular nucleus, have been observed in animals supplemented with chitosan [ 23 ]. Recognizably, PPAR activation in patients with type 2 diabetes enhances insulin and glucose levels [ 42 ]. Such a result may be associated with the appetite-suppressing properties of chitosan, as our study provides evidence that chitosan supplementation can modulate appetite-related hormones. We observed a significant increase and decrease in adiponectin and leptin levels, respectively, which reached both within-group and between-group differences. Taken together, many animal studies furnish the role of leptin, adiponectin, and NPY in appetite modulation and systematic effects on obesity. Serum leptin concentration and its receptor expression in adipose tissue increased in chitosan fed-pigs compared with animals receiving basal diet, and increased expression of NPY in the hypothalamic nuclei and in the jejunum [ 23 ]. In addition to the anorexigenic i. The main strength of this study is the RCT design and novelty, given that this study was the first human research that examined the effects of chitosan supplementation on adolescents with overweight or obesity. However, our studies have limitations that serve as perspectives. First, we did not assess body fat, which should preferably be examined by a reliable method. Second, although we measured some appetite-related hormones, we did not assess ghrelin levels or perform appetite questionnaires. Third, we encourage further RCT to assess these markers, as well as crossover acute studies using transabdominal ultrasound to allow the examination of the gastric emptying on meal tests with and without chitosan supplementation. At last, further research is also required to better understand the effects of chitosan on the gut microbiota, as well as on advanced biomarkers of the lipid profile e. In general, our study showed that chitosan supplementation can improve cardiometabolic parameters anthropometric indicators of obesity and lipid and glycemic markers and appetite-related hormones adiponectin, leptin, and NPY in adolescents with overweight or obesity. However, the effects must be considered as an adjuvant instead of a magic bullet for the management of obesity. Preferably, such a strategy ought to be planned mainly in combination with a hypocaloric diet and physical exercise supervised by proper heathy professionals. Farzad Shidfar, Department of Nutrition, School of Public Health, Iran University of Medical Sciences, Tehran, Iran. E-mail: shidfar. f iums. Fryar CD, Carroll MD, Ogden CL. Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2—19 years: United States, — through —; Google Scholar. Flegal KM, Wei R, Ogden C. Weight-for-stature compared with body mass index—for-age growth charts for the United States from the Centers for Disease Control and Prevention. Am J Clin Nutr. Article CAS PubMed Google Scholar. Boyer BP, Nelson JA, Holub SC. Childhood body mass index trajectories predicting cardiovascular risk in adolescence. J Adolesc Health. Article PubMed PubMed Central Google Scholar. Baker JL, Olsen LW, Sørensen TI. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med. Article CAS PubMed PubMed Central Google Scholar. Simmonds M, Burch J, Llewellyn A, Griffiths C, Yang H, Owen C, et al. The use of measures of obesity in childhood for predicting obesity and the development of obesity-related diseases in adulthood: a systematic review and meta-analysis. Health Technol Assess Winchester, England. Article Google Scholar. Cali AM, Caprio S. Obesity in children and adolescents. J Clin Endocrinol Metab. Baños RM, Cebolla A, Botella C, García-Palacios A, Oliver E, Zaragoza I, et al. Improving childhood obesity treatment using new technologies: the ETIOBE system. Clin Pract Epidemiol Mental Health. Santos HO, Lavie CJ. Weight loss and its influence on high-density lipoprotein cholesterol HDL-C concentrations: a noble clinical hesitation. Clin Nutr ESPEN. Article PubMed Google Scholar. Santos HO, Macedo RC. Impact of intermittent fasting on the lipid profile: assessment associated with diet and weight loss. Field AE, Austin S, Taylor C, Malspeis S, Rosner B, Rockett HR, et al. Relation between dieting and weight change among preadolescents and adolescents. Fisher JO, Birch LL. Restricting access to palatable foods affects children's behavioral response, food selection, and intake. Kirby M, Danner E. Nutritional deficiencies in children on restricted diets. Pediatr Clin. García OP, Long KZ, Rosado JL. Impact of micronutrient deficiencies on obesity. Nutr Rev. Lee DPS, Peng A, Taniasuri F, Tan D, Kim JE. Impact of fiber-fortified food consumption on anthropometric measurements and cardiometabolic outcomes: a systematic review, meta-analyses, and meta-regressions of randomized controlled trials. Crit Rev Food Sci Nutr. Ranaivo H, Thirion F, Béra-Maillet C, Guilly S, Simon C, Sothier M, et al. Increasing the diversity of dietary fibers in a daily-consumed bread modifies gut microbiota and metabolic profile in subjects at cardiometabolic risk. Gogolewski, S. Bioresorbable polymers in trauma and bone surgery. Injury , 31 Suppl 4 : 28— Hejazi, M. Chitosan-based gastrointestinal delivery systems. Journal of Controlled Release , 89 2 : — Hoppe-Seiler, F. Chitin and chitosan. Berichte der Deutschen Chemischen Gesellschaft , 27 : — Huang, R. Dietary oligochitosan supplementation enhances immune status of broilers. Journal of the Science of Food and Agriculture , 87 1 : — Huang, Y. Degradation of chitosan by hydrodynamic cavitation. Polymer Degradation and Stability , 98 1 : 37— Ikada, Y. Biodegradable polyesters for medical and ecological applications. Macromolecular Rapid Communications , 21 3 : — Jiang, M. The promotion of peripheral nerve regeneration by chitooligosaccharides in the rat nerve crush injury model. Neuroscience Letters , 3 : — Jikakis, J. Chitin, Chitosan and Related Enzymes. Academic Press, New York, pp. Kean, T. Biodegradation, biodistribution and toxicity of chitosan. Advanced Drug Delivery Reviews , 62 1 : 3— Kong, X. New biodegradable small-diameter artificial vascular prosthesis: A feasibility study. J ournal of Biomedical Materials Research Part A , A 6 : — Madihally, S. Porous chitosan scaffolds for tissue engineering. Biomaterials , 20 12 : — Moon, D. Cyclic mechanical preconditioning improves engineered muscle contraction. Tissue Engineering Part A , 14 4 : — Muzzarelli, R. Natural Chelating Polymers. Pergamon Press, New York, 83— Notin, L. Morphology and mechanical properties of chitosan fibers obtained by gel-spinning: Influence of the dryjet-stretching step and ageing. Acta Biomaterialia , 2 4 : — Onishi, H. Biodegradation and distribution of water-soluble chitosan in mice. Biomaterials , 20 : — Park, J. Synthesis and characterization of sugar-bearing chitosan derivatives: Aqueous solubility and biodegradability. Biomacromolecules , 4 4 : — Rinaudo, M. Chitin and chitosan: Properties and applications. Progress in Polymer Science , 31 7 : — Sinha, V. Chitosan microspheres as a potential carrier for drugs. International Journal of Pharmaceutics , : 1— Tsaih, M. Effects of removing small fragments with ultrafiltration treatment and ultrasonic conditions on the degradation kinetics of chitosan. |
| Access options | S Chitosan for metabolism to data collection and manuscript metabklism. Noticeably, a large amount of Akkermansia muciniphila was enriched in both high-fat or low-fat fo groups. The role metabolusm dietary Beta-alanine and muscle fatigue delay in the development Hydration for bladder health treatment Hydration for bladder health childhood obesity. Khor E, Lim LY Implantable applications of chitin and chitosan. In vivo and ex vivo imaging showed that maximal luciferase activities were detected in brain and gastrointestinal tract. Although there is a statistically significant reduction, this has not produced any clinically relevant adverse effects over a period of 90 days. Audio-video recording of the entire informed consent process was carried out according to the schedule Y of Indian GCP. |
Unbedingt, er ist recht
Sie lassen den Fehler zu. Ich kann die Position verteidigen. Schreiben Sie mir in PM, wir werden besprechen.
Was Sie mir beraten?
Es ist die richtigen Informationen
Mir scheint es der ausgezeichnete Gedanke