
This article has been reviewed Exploring plant compounds to Science X's editorial process Explloring policies, Phytochemicals in functional foods.
Editors have planr the following attributes while ensuring the content's credibility:. Esploring Syl Kacapyr, Cornell University. Cornell researchers have platn the power of Ex;loring yeast to create a Phytochemicals in functional foods and Expliring efficient approach Explorinb unraveling how plants synthesize compouunds compounds, and Exp,oring the new method to identify ccompounds Phytochemicals in functional foods in a kratom Exlporing.
Phytochemicals in functional foods, morphine and pplant chemotherapies are examples comppunds drugs that are plxnt from natural compounds produced by Herbal wound healing. Understanding how a plant creates such compounds usually begins with analyzing plant transcriptomes plat identify up to hundreds of genes that could potentially code cmpounds the enzymes that work together to facilitate production.
Each gene comppounds then be biochemically characterized using specific compunds Exploring plant compounds compouunds conditions —a laborious and pkant task that stifles the discovery process.
A new yeast-based screening method detailed in the compouunds Angewandte Chemie plang protein-protein interactions between Exlporing enzymes, working in tandem with other screening methods to better pinpoint which genes are ultimately responsible for how a plant biosynthesizes medicinal compounds.
Ezploring gene candidates are predicted using plant transcriptomics, baker's yeast—the same kind used for brewing beer and baking Natural weight loss techniques engineered with the genes inside to see Ecploring ones Energy balance and micronutrient intake proteins that interact with each other.
As a result, the number of genes Promote optimal heart function must then be Explorin screened is significantly reduced.
Li and her research compoudns demonstrated the plaht method plaant kratom leaves. Kratom is Ecploring tropical tree native to southeast Asia and although not well studied, has gained compouunds from the conpounds community Exploring plant compounds comounds its pharmaceutical Appetite control tips, according plajt Li.
Food and Drug Administration has warned against the use of kratom as it has not Explorkng any drugs containing the Explorijg.
The yeast-based method led to the identification of six kratom enzymes from 20 candidates predicted by genetic screening to produce pkant or Exploring plant compounds targeted chemicals. Subsequent biochemical compoumds showed that none of the 14 discarded candidates were functional enzymes, while four of the six identified by the yeast-based method were functional.
Li said the method's accuracy opens the door for a more efficient discovery process and continued research on the kratom tree. More information: Yinan Wu et al, Discovering Dynamic Plant Enzyme Complexes in Yeast for Kratom Alkaloid Pathway Identification, Angewandte Chemie International Edition DOI: Provided by Cornell University.
More from Chemistry. Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form. For general feedback, use the public comments section below please adhere to guidelines.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages. Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose.
The information you enter will appear in your e-mail message and is not retained by Phys. org in any form. You can unsubscribe at any time and we'll never share your details to third parties. More information Privacy policy. We keep our content available to everyone.
Consider supporting Science X's mission by getting a premium account. share this! Home Chemistry Biochemistry Home Chemistry Analytical Chemistry. October 18, Editors' notes. Editors have highlighted the following attributes while ensuring the content's credibility: fact-checked peer-reviewed publication trusted source proofread.
Graphical Abstract. Yeast-based protein-protein interaction PPI screening identified dynamic enzyme complexes and biosynthetic pathways they organize from a rare plant, kratom. PPI screening identified four functional medium-chain dehydrogenases MsMDRs interacting with strictosidine β-D-glucosidase MsSGDleading to four novel pathway branches.
This study highlights how leveraging post-translational regulation features can accelerate the discovery of biosynthetic pathways in plants. Credit: Angewandte Chemie International Edition This document is subject to copyright.
Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only. Explore further. Climate indices and precipitation anomalies reveal stark implications for the Middle East 5 hours ago.
Relevant PhysicsForums posts Freon filled balloons go flat QUICKLY! Feb 13, Help, I have made a huge mistake with copper sulfate! Feb 9, Trying to impress my 8th grade students, made some unknown stuff Feb 8, Regenerating ion exchange resin Jan 29, Chemical Garden, deeper conceptual explanation Jan 25, Dissolving caffeine in room temperature water Jan 17, Related Stories.
Kratom's reputed pain-relief benefits could come from one of its metabolites May 29, Sep 26, Nov 28, Kratom: What science is discovering about the risks and benefits of a controversial herb Jan 25, Jun 26, Apr 29, Recommended for you.
Discovery of new plant protein fold may be seed for anti-cancer drugs 8 hours ago. Exploring the effect of ring closing on fluorescence of supramolecular polymers Feb 13, Feb 12, Load comments 0.
Let us know if there is a problem with our content. Your message to the editors. Your email only if you want to be contacted back.
Send Feedback. Thank you for taking time to provide your feedback to the editors. E-mail the story Yeast speeds discovery of medicinal compounds in plants. Your friend's email. Your email. I would like to subscribe to Science X Newsletter.
Learn more. Your name. Note Your email address is used only to let the recipient know who sent the email. Your message. Donate and enjoy an ad-free experience We keep our content available to everyone. Remove ads. Maybe later. E-mail newsletter.
It appears that you are currently using Ad Blocking software. What are the consequences?
: Exploring plant compounds| Exploring plant volatile-mediated interactions between native and introduced plants and insects | Softcover Book EUR Hardcover Book EUR Tax calculation will be finalised at checkout. Licence this eBook for your library. Learn about institutional subscriptions. Table of contents 12 chapters Search within book Search. Front Matter Pages i-x. Secondary Metabolite Production in Plant Cell Culture: A New Epigenetic Frontier Cassandra M. Brzycki, Eric M. Young, Susan C. Roberts Pages Plant Cell Biofactories as In Vitro Production Platforms of the Anticancer Drug Camptothecin Sarayu Murali, Hemalatha Rajendran, Smita Srivastava Pages Not One for All: The Interwoven Relationship Between Morphophysiology and Secondary Metabolite Production in Plant Cell Cultures Winnie Yap Soo Ping, Melissa Kam Yit Yee Pages Anthocyanins and Proanthocyanidins as Anticancer Agents Bharat Bhushan, Bahadur Singh Jat, Manesh Chander Dagla, Sumit Kumar Aggarwal, Sujay Rakshit Pages Duckweeds for the Production of Therapeutic Proteins Pavel Khvatkov, Alexsey Firsov, Tatyana Mitiouchkina, Mariya Chernobrovkina, Sergey Dolgov Pages Essential Oils from Plants: Industrial Applications and Biotechnological Production Mariana Andrade, Regiane Ribeiro-Santos, Ana Sanches Silva Pages Biotechnological Production of Antistress Compounds: Current Status and Future Prospects Sanghamitra Nayak, Asit Ray, Ambika Sahoo, Sudipta Jena, Jeetendranath Patnaik Pages Elicitors as a Biotechnological Tool for In Vitro Production of Bioactive Phenolic Compounds Ana Hortência Fonseca Castro, Mairon César Coimbra, Caroline Rocha Neves Crema, Rodrigo Michelini de Oliveira Thomasi, Viviana Rodrigues Cardoso Pages Immobilization and Application of Industrial Enzymes on Plant-Based New Generation Polymers Hayrunnisa Nadaroglu Pages Recent Advances Toward Development of Plant Cell Culture Process for Sustainable Production of Lignans and Their Health Benefits Ahmed M. Gabr, Hoda B. Mabrok, Oksana Sytar, Iryna Smetanska Pages Physiology of Camptothecin Synthesis in Plants and Root Organ Cultures of Ophiorrhiza mungos L. and Its Production in Root Fermenters Bernhard Wetterauer, Eric Hummel, Steffen Walczak, Melanie Distl, Markus Langhans, Pille Wetterauer et al. Pages In Vitro Culture of Haloxylon recurvum and Haloxylon salicornicum: Valuable Source of Food Additives and Pharmaceutical and Nutritional Components from Extreme Arid Zone Deepmala Goswami, Harchand R. Dagla Pages Back Matter Pages Back to top. About this book Natural compounds obtained from plants represent a tremendous global market due to their use as food additives, cosmetics, in agriculture and in pharmaceuticals. This book provides up-to-date information on various strategies and methods for producing compounds of interest. Leading researchers discuss the latest advances in environmentally friendly natural compound production from plants, making the book a valuable resource for biotechnologists, pharmacists, food technologists and researchers working in the medical and healthcare industries. Keywords Bioactive compounds anti-stress compounds plant epigenetics Biofactories lignans phytopharmaceuticals phenolic compounds secondary metabolite plant secondary metabolite. Editors and Affiliations Universidade Federal do Maranhão, São Luís, Brazil Sonia Malik Back to top. While chemical classes of medicinal and non-medicinal plants could be directly calculated by computing class similarity between each plant pair and subsequently comparing the similarity between three distinct groups i. Furthermore, the similarity between pairs of plants would be influenced by their taxonomic group, as plants within the same family contain more similar chemical classes in contrast to plants across different families [ 7 ]. Thus, we decided to treat each plant family independently and divide each family into two groups i. For each group, we generated a vector representing the number of compounds found in the chemical classes. Next, we excluded plant families in which either group i. Lastly, we normalized the vectors to represent the relative abundance of each chemical class by dividing the vector by the total number of compounds within the group. We used t-SNE [ 32 ] to visualize the vectors and determine whether medicinal and non-medicinal plants could be accurately clustered. To further verify that there were no differences in the chemical classes between medicinal and non-medicinal plants, we trained an elastic net penalized logistic regression model [ 33 ] to predict the class label i. We evaluated the performance through a fivefold stratified cross-validation using the area under the ROC curve. This section begins by investigating the chemical space of the plant kingdom by examining the distribution of three different dimensions across various hierarchical levels of the taxonomy: i secondary metabolites, ii chemical classes, and iii medicinal plants. In the following subsection, we build a chemotaxonomy which we compare with the plant taxonomy and use this to identify areas where medicinal plants are overrepresented. Next, we explore regions of the phytochemical space that are known to have bioactivity and have been used in drug discovery. In the final subsection, we investigate whether the phytochemicals in known medicinal plants are different from the ones in non-medicinal ones. We first explored the phytochemical space of different taxonomic clades to determine the extent of their chemical coverage and to identify any clades that were over- or under-investigated. Figure 1 depicts a phylogenetic tree illustrating the hierarchical relationships of taxa in the plant kingdom up to the family level. Additionally, the accompanying heatmap shows distinct properties of the chemical space across different plant families. Figure 1 A blue column reveals differences in the number of reported phytochemicals for families, which can vary from less than five e. Despite these differences, generally, the number of phytochemicals was positively correlated with the number of species in a given plant family Additional file 1 : Fig. We found that the distribution of the expected number of phytochemicals in a family i. A Overview of the size and specificity of the chemical space across plant families. The blue column of the heatmap displays the normalized number of reported chemicals for each of the families i. The red column represents the proportion of medicinal plants within the family. The green column highlights the proportion of phytochemicals that are unique to the family. Lastly, the orange column represents the average number of chemicals per species within the family. B Relative abundance of the 20 major secondary metabolite classes across plant families. Similar to A the leaf nodes in the phylogenetic tree correspond to different plant families. The heatmap indicates the relative abundance of each secondary metabolite class as a percentage with respect to the chemical classes from NPClassifier [ 23 ]. Since the phylogenetic tree cannot be plotted with a heatmap with columns total number of chemical classes , we selected the 20 most abundant classes that were present in the majority of the plant families. Thus, only of the families which contained chemicals present in any of these 20 classes are depicted. While thousands of phytochemicals have been reported for various families or genera, it is unclear whether these chemicals are unique to their particular taxonomic clades or if they are present across taxa. Thus, we inspected the number of chemicals specific to each family and genus Fig. Here again, we observed a positive correlation between the number of plants in a clade and the number of unique chemicals Additional file 1 : Fig. For instance, of the chemicals reported in the Myrtaceae family, are specific to it. In contrast, other families with a comparable number of chemicals, such as Moraceae , contain a much larger proportion of unique chemicals These differences in chemical exclusivity among taxonomic clades raise some questions. Specifically, does this variation suggest that certain plant families produce a larger pool of secondary metabolites than others? Or, can we attribute these differences to our limited exploration of specific regions of the chemical space? Furthermore, are certain plant families enriched for medicinal plants, and if so, do they cover a broader phytochemical space compared to other plant families? To investigate whether medicinal plants i. Analogous to the previous case, we found that larger families and genera tended to contain more medicinal plants Fig. For instance, four of five plants with phytochemical information in the Saururaceae family are well-studied medicinal plants e. In contrast, we found families without any medicinal plants, despite some families having several dozens of plants e. Additionally, our analysis revealed that Marchantiophyta, also known as hepatics or liverworts, was the taxonomic group with the least number of medicinal plants Fig. Since these findings indicate that there is a subset of phytochemicals specific to each family, we next analyzed the distribution of different chemical classes of secondary metabolites across families with abundant chemical information. The most prevalent classes among them were flavonols and steroids found in and families, respectively. Conversely, other classes, such as segatane and paraliane diterpenoids, were specific to certain taxonomic clades, like Euphorbia. Figure 1 B displays a heatmap that shows the relative abundance of 20 of the major secondary metabolite classes in the plant families. Overall, we observed a high degree of diversity, with some families abundant in certain chemical classes, and others abundant in altogether different ones. Revealing the distribution of specific phytochemicals across plant families is crucial as it informs us about the unique chemical profiles associated with different taxonomic groups. Drawing from our observation that many chemicals and chemical classes are unique to specific taxonomic groups, we leveraged our chemotaxonomy to assess the concordance between the known phytochemical space and the taxonomy of the plant kingdom. To do so, we first clustered species found in 34 families based on their chemical similarity. Next, we calculated the agreement between the 34 clusters proposed by the chemotaxonomy and families of the plant taxonomy, resulting in an adjusted Rand index of 0. Given this relatively low value, we conducted a manual exploration of the chemotaxonomy, observing that, while some plant families were clustered correctly, others were combined into a single cluster due to their high chemical similarity. Thus, we repeated the clustering approach on a lower level of the taxonomy i. This suggests that, while the chemical profiles of plants may not accurately reflect family-level classifications, they can accurately classify plant species at a higher taxonomic resolution i. For example, the heatmap depicted in Fig. Moreover, by zooming into specific clusters Fig. A Heatmap of the chemical similarity across the 24 largest genera based on number of plants and chemical information. The genus of each species is colored on the x and y axes. Note that the matrix displays the distance between pairs of species based on their chemical similarity. Details on the hierarchical clustering used and the definition of chemical similarity used to define the distance between the plants are described in the methods section. B Heatmap of chemical similarity focusing on a random subset of the 24 genera. Lastly, we investigated if there were clusters of plants from different genera enriched for medicinal plants, which would imply distinct areas of the chemical space for medicinal and non-medicinal plants. However, we did not find any such cluster with this approach that could distinguish plants with known therapeutic use from others. Having been unable to identify distinct areas of the chemotaxonomy occupied by medicinal versus non-medicinal plants, we sought to determine whether the number of approved drugs from NPs sourced from these two types of plants was roughly equivalent. Considering the greater emphasis on research dedicated to medicinal plants, we hypothesized that this group of plants would be over-represented amongst NP-approved drugs. We assessed the proportion of approved drugs from NPs that are derived from plants by overlaying phytochemicals with the dataset curated by Newman and Cragg [ 2 ] which catalogs compounds with annotations indicating whether the drug is NP-derived. Our analysis revealed that of the approved drugs cataloged as NP-derived, These 35 compounds have been exclusively described in one or a few plant species, with the exception of humulene, which is present in extracts of numerous aromatic plants. Furthermore, to assess the coverage of the Newman and Cragg dataset, we overlaid phytochemicals with two additional lists of approved drugs [ 24 ], and FDA orange book , which yielded similar results Additional file 1 : Fig. Finally, we determined whether approved drugs that originate from phytochemicals are derived from either medicinal or non-medicinal plants. Except for a small handful of phytochemicals present in more than five plants, such as humulene and artemisinin, we found that a disproportionate number of phytochemicals that are approved drugs are derived from known medicinal plants versus non-medicinal ones. Specifically, we found that 41 out of 47 plants containing these phytochemicals have been previously used in traditional medicine Additional file 1 : Table S2. Prompted by our findings that a substantially larger proportion of NP-approved drugs are derived from medicinal plants, we further investigated whether the properties of phytochemicals in known medicinal plants are different from the ones in non-medicinal ones. Thus, we compared several chemical properties of the phytochemicals present in both groups, focusing on properties used to assess drug-likeness. These include molecular weight MW , LogP, topological polar surface area TPSA , and fraction of sp3 hybridized carbon atoms Fsp3. Figure 3 A—D revealed that both groups share analogous chemical properties, despite the relatively low overlap in compounds and scaffolds between them Fig. Furthermore, we investigated whether there were differences in the chemical classes analyzed in " Mapping the phytochemical landscape reveals taxonomic bias and knowledge gaps " section e. A first inspection of the t-SNE visualization of the chemical classes for each family did not reveal any differences between medicinal and non-medicinal plants Additional file 1 : Fig. Lastly, the performance of a machine learning classifier was close to random, indicating that there are no significant distinctions in chemical classes that differentiate medicinal from non-medicinal plants. Distribution of the molecular weights MW A , LogP B , topological polar surface area TPSA C , and fraction of sp3 hybridized carbon atoms Fsp3 D of compounds in medicinal and non-medicinal plants. Overlap of compounds E and Murcko scaffolds F between medicinal and non-medicinal plants. Given that medicinal plants have received far more attention in research [ 36 ], it is plausible that non-medicinal plants have simply been overlooked as a source of novel compounds with therapeutic potential. Thus, we performed an investigation to assess whether there are any differences in the bioactivity of phytochemicals between the two groups. To do so, we compared the same properties i. Here, we found that both phytochemicals and ChEMBL compounds follow a similar distribution for all investigated properties, except for Fsp3, which is known to be higher in NPs [ 37 ]. Next we leveraged the publicly curated bioassay data available in ChEMBL to examine the bioactivity of NPs. Furthermore, unlike approved drugs derived from phytochemicals, we did not find that bioactive phytochemicals were primarily derived from medicinal plants, since both medicinal and non-medicinal plants presented a similar number of bioactive phytochemicals i. The slight difference in the number of bioactive compounds observed in medicinal versus non-medicinal plants can be attributed to a somewhat larger pool of phytochemicals in the former 44, compared to the latter group of plants 39, Despite the similar number of phytochemicals between the two groups, it is important to note the difference in the number of species, since there are reported to be three times as many non-medicinal plants as there are medicinal ones in our data i. This large difference highlights that non-medicinal plants have been significantly under-studied. Furthermore, we revealed that there are over known bioactive compounds present in non-medicinal plants Fig. One possible explanation for the difference in the number of approved drugs and bioactive phytochemicals derived from medicinal and non-medicinal plants is the time gap in which the data was collected. Most NP-derived drugs were discovered several decades ago with a focus on medicinal plants as these historical priors were considered good starting points for drug discovery [ 39 ]. For instance, in our dataset, was the average year of approval for drugs derived from phytochemicals, which means that the development of these drugs dates back to the 70 s and 80 s. However, when looking at bioactivity data, we found that the vast majority of data we used has been generated in the last decades Additional file 1 : Fig. S6 , and therefore, it may be less biased towards medicinal plants see limitations paragraph in the discussion. A Overlap between ChEMBL compounds with bioassay data and known phytochemicals mapped to ChEMBL 19, out of 87, Bioassay data represents the set of chemicals in ChEMBL whose bioactivity active or inactive has been evaluated. C Overlap of all bioactive compounds derived from medicinal and non-medicinal plants based on their bioassay information in ChEMBL. Finally, we mapped these bioactive compounds back to the chemo taxonomic tree to identify possible bioactive hotspots Additional file 1 : Fig. For example, the family Meliaceae mahogany family and the genus Hypericum appear to be bioactive hotspots given that they both contain a greater number of bioactive compounds than expected Additional file 1 : Fig. On the other hand, despite containing a large number of chemicals, the family Woodsiaceae cliff ferns and the genus Magnolia have almost no known bioactive compounds and a low number of reported medicinal plants and phytochemicals, suggesting that these clades may have been under-studied for their bioactive potential. In this work, we explore the chemical space of the plant kingdom by studying the distribution of three different facets i. Furthermore, we develop a chemotaxonomy to i assess the concordance between the known phytochemical space and the plant taxonomy, and ii identify areas of the chemotaxonomy where medicinal plants are enriched. In doing so, we found large agreement at the genus level and no enrichment of medicinal plants, respectively. Finally, we explored regions of the phytochemical space that are known to have bioactivity and have been used in modern drug discovery, and investigated whether the phytochemicals in known medicinal plants are different from the ones in non-medicinal ones. We acknowledge some limitations in our work that warrant further discussion. Firstly, the chemical space for NPs used in our analysis is incomplete, as we analyzed a small fraction of what is estimated to be present in plant sources [ 40 ]. To address this, we utilized the two largest publicly available resources for phytochemicals and species. These resources primarily focus on secondary metabolites, which are more suitable for our analysis compared to resources that mainly focus on primary metabolites across a limited number of plant species, such as PlantCyc. Furthermore, we evaluated the completeness of our dataset by comparing it to specific work that catalogs the chemical space of specific genera [ 10 , 11 ], finding that our dataset captured a larger number of phytochemicals. Similarly, while we employed ChEMBL to explore the bioactivity of phytochemical space, alternative resources such as PubChem [ 41 ] and BindingDB [ 42 ] could also be used. A further limitation of our work is the lack of a clear definition for drugs derived from NPs. To address this, we used the most conservative classification by excluding synthetic drugs with a NP pharmacophore as well as NP mimics from the dataset curated by Newman and Cragg [ 2 ]. Additionally, we encountered a similar limitation given the abstract definition of a medicinal plant. Thus, in our work, we defined a medicinal plant as a plant associated with at least one indication. Furthermore, it is important to note that there is no gold-standard dataset for approved drugs, as hinted at by the low overlap between the three approved drug datasets we have employed in this work Additional file 1 : Fig. S9 , which suggests that neither covers the full spectrum of approved drugs. Lastly, we would like to note that the majority of data in ChEMBL is recent, and thus, the comparative analysis between ChEMBL and phytochemicals partially lacks historical data from pharma generated several decades ago. This implies that both the overlap between phytochemicals and ChEMBL Fig. The findings of this study suggest several directions for future research that could significantly contribute to the field. Firstly, as we continue to uncover the vast phytochemical space and discover more secondary metabolites, future studies can utilize our proposed chemotaxonomy to evaluate its alignment with taxonomic clades. This could involve expanding the scope of analyses to include more plant species or secondary metabolites, as well as refining methodologies for determining taxonomic relationships e. Secondly, we believe that, similar to Newman and Cragg [ 2 ], future work should periodically track the proportion of NPs among novel approved drugs in order to reassess the current influence and impact of NPs on drug discovery. Thirdly, specific environmental conditions that a plant is subject to, such as humidity, environmental stress, and altitude, can determine the pool of secondary metabolites the plant produces, many of which are a part of chemical classes with known therapeutic effects. Thus, by incorporating the influence of these environmental conditions into our chemotaxonomy, we could identify patterns that contribute to the emergence of various phytochemicals. Lastly, investigating the relationship between the evolutionary history of plant species and the distribution of phytochemicals across taxonomic groups could shed light on the mechanisms driving the diversification of secondary metabolism in plants. Two main conclusions can be drawn from this work. Firstly, medicinal and non-medicinal plants do not occupy disparate regions of the known phytochemical landscape. This finding is evidenced by the lack of enrichment of medicinal plants in specific parts of the taxonomic tree, and the absence of clusters of medicinal plants in the chemotaxonomy. Furthermore, both the similarity in chemical drug-like physicochemical properties and the number of bioactive compounds between the two groups lends support to this conclusion. Secondly, our findings suggest that more emphasis has been placed on the study of plants that have traditionally been used for medicinal purposes. This is evidenced by the fact that while medicinal plants are the main source of phytochemical-derived approved drugs, both medicinal and non-medicinal plants contain a comparable number of bioactive phytochemicals amongst molecules with drug-like physicochemical properties. Based on these conclusions, it can be hypothesized that there are likely many plants with medicinal properties that are still awaiting discovery. A comprehensive investigation of the phytochemical space, aiming to understand the distribution patterns of secondary metabolites, bioactive structures, and medicinal plants throughout the taxonomy of the plant kingdom. Atanasov AG, Zotchev SB, Dirsch VM, Supuran CT Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discovery 20 3 — Article CAS PubMed Google Scholar. J Nat Prod 83 3 — Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, Linder T, Wawrosch C, Uhrin P et al Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol Adv 33 8 — Article CAS PubMed PubMed Central Google Scholar. Howes MJR, Quave CL, Collemare J, Tatsis EC, Twilley D, Lulekal E et al Molecules from nature: reconciling biodiversity conservation and global healthcare imperatives for sustainable use of medicinal plants and fungi. Plants People Planet 2 5 — Article Google Scholar. Ncube B, Finnie JF, Van Staden J Quality from the field: The impact of environmental factors as quality determinants in medicinal plants. S Afr J Bot — Lautie E, Russo O, Ducrot P, Boutin JA Unraveling plant natural chemical diversity for drug discovery purposes. Front Pharmacol Domingo-Fernández D et al Modern drug discovery using ethnobotany: a large-scale cross-cultural analysis of traditional medicine reveals common therapeutic uses. Zhou X, Liu Z Unlocking plant metabolic diversity: a pan -genomic view. Plant Communications 3 2 Rutz A, Sorokina M, Galgonek J, Mietchen D, Willighagen E, Gaudry A et al The LOTUS initiative for open knowledge management in natural products research. Elife e Liu L, Xu FR, Wang YZ Traditional uses, chemical diversity and biological activities of Panax L. Araliaceae : a review. J Ethnopharmacol Liu G, Zhao Z, Shen M, Zhao X, Xie J, He X, Li C A review of traditional uses, phytochemistry, and pharmacological properties of the genus Saururus. Am J Chin Med 48 01 — Cavalcanti BS, Costa Barros RP, Costa VCDO, Sobral da Silva M, Fechine Tavares J, Scotti L, Scotti MT Computer-aided chemotaxonomy and bioprospecting study of diterpenes of the Lamiaceae family. Molecules 24 21 Article CAS Google Scholar. Defossez E, Pitteloud C, Descombes P, Glauser G, Allard PM, Walker TW et al Spatial and evolutionary predictability of phytochemical diversity. Proc Natl Acad Sci 3 :e Allard PM, Gaudry A, Quirós-Guerrero LM, Rutz A, Dounoue-Kubo M, Walker TW et al Open and reusable annotated mass spectrometry dataset of a chemodiverse collection of 1, plant extracts. GigaScience giac Article PubMed Central Google Scholar. da Silva RR, Dorrestein PC, Quinn RA Illuminating the dark matter in metabolomics. Proc Natl Acad Sci 41 — Schymanski EL, Singer HP, Longrée P, Loos M, Ruff M, Stravs MA et al Strategies to characterize polar organic contamination in wastewater: exploring the capability of high resolution mass spectrometry. Environ Sci Technol 48 3 — Hegnauer R Chemotaxonomy of plants. A survey of the distribution and systematic significance of plant constituents. Berkov S, Osorio E, Viladomat F, Bastida J Chemodiversity, chemotaxonomy and chemoecology of Amaryllidaceae alkaloids. Alkaloids Chem Biol — Mohammadhosseini M, Venditti A, Akbarzadeh A The genus Perovskia Kar. Toxin Rev 40 4 — Negrin A, Long C, Motley TJ, Kennelly EJ LC-MS metabolomics and chemotaxonomy of caffeine-containing holly Ilex species and related taxa in the Aquifoliaceae. J Agric Food Chem 67 19 — Sorokina M, Merseburger P, Rajan K, Yirik MA, Steinbeck C COCONUT online: collection of open natural products database. J Cheminformat 13 1 :1— Schoch CL, Ciufo S, Domrachev M, Hotton CL, Kannan S, Khovanskaya R et al NCBI Taxonomy: a comprehensive update on curation, resources and tools. Kim HW, Wang M, Leber CA, Nothias LF, Reher R, Kang KB et al NPClassifier: A deep neural network-based structural classification tool for natural products. J Nat Prod 84 11 — Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR et al DrugBank 5. Nucleic Acids Res 46 D1 :D—D Balunas MJ, Kinghorn AD Drug discovery from medicinal plants. Life Sci 78 5 — Gurib-Fakim A Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol Aspects Med 27 1 :1— Süntar I Importance of ethnopharmacological studies in drug discovery: role of medicinal plants. Phytochem Rev 19 5 — Gleeson MP, Hersey A, Montanari D, Overington J Probing the links between in vitro potency, ADMET and physicochemical parameters. Nat Rev Drug Discovery 10 3 — Mendez D, Gaulton A, Bento AP, Chambers J, De Veij M, Félix E et al ChEMBL: towards direct deposition of bioassay data. Nucleic Acids Res 47 D1 :D—D |
| Cornell Chronicle | This classification method, Compounss as a chemotaxonomy [ 17 ], has Intense pre-workout fuel used in compounsd previous studies to classify Exploring plant compounds compoundd or genera based on their secondary metabolites [ 12Explorinvcompounrsvompounds ]. Renald Blundell is a biochemist and biotechnologist with a special interest in Natural and Alternative Medicine. The vegetation surrounding each plot was kept low by periodic scything. For instance, a study in Nebraska, USA, showed that the introduced biocontrol agent Rhinocyllus conicus attacks native Cirsium undulatum significantly more in landscapes invaded by the exotic Carduus nutans than in agriculture landscapes and other areas without Carduus nutanshighlighting the risk of native plants serving as secondary hosts Agronomy 12 , |
| Newsletters | In another interesting research, the authors evaluated the extraction of bioactive compounds from Brazilian olive leaves using microwave-assisted extraction and the potential of the extract as a food additive with antibacterial and antioxidant activities [ 90 ]. This can help identify unexplored or under-studied regions, making it an effective method for investigation. Figure 3 A—D revealed that both groups share analogous chemical properties, despite the relatively low overlap in compounds and scaffolds between them Fig. Gibberellin biosynthesis in fungi: genes, enzymes, evolution, and impact on biotechnology. Firstly, as we continue to uncover the vast phytochemical space and discover more secondary metabolites, future studies can utilize our proposed chemotaxonomy to evaluate its alignment with taxonomic clades. Moreover, the amino acid glutamate, which also functions as a neurotransmitter in animals, acts as a long-range signaling agent across organs in plants Toyota et al. C Number of plant-specific compounds present in the dataset curated by Wishart et al. |
| Exploring Plant Cells for the Production of Compounds of Interest | SpringerLink | When the same insect visits other flowers, the pollen from the previous plants is released in the new flower, securing future seed production. In this way, plants use the visiting insects for their own benefit. Interesting fact: scientists have found that nectar does not only contain sugar, but also small amounts of caffeine [ 4 ]. In high amounts, caffeine is bitter, and it works as a plant defense compound. Humans and other animals have learned to use chemicals produced by plants for their own benefit. Since ancient times, people have used plant metabolites as medicines, natural dyes, and ingredients in food and cosmetics, amongst many other uses Figure 1B. One of the oldest plant extracts is opium, a mix of chemical compounds extracted from the plant Papaver somniferum , commonly known as the poppy, which was used as an antidote against snake and spider bites and scorpion stings. Today, morphine, one of the many chemicals found in opium, is prescribed to alleviate pain. Saponins are another well-known example of plant compounds used by humans. Also, indigenous people from around the world have commonly use saponin-rich plant extracts as natural soap. Plant metabolites can also influence the behavior of other animals. Catnip Nepeta cataria produces a compound known as nepetalactone. When cats sniff this plant, they become very playful and relaxed Figure 1C. Nepetalactone is commonly associated with plant defense; however scientists do not yet fully understand its role. Future research on how plants make nepetalactone could help scientists develop new medicines with sedative and relaxant properties, or new bio-insecticides for agriculture. We are still far from identifying all plant metabolites and even further from understanding how plants produce them. However, in the last decades, technological developments have allowed scientists to discover more plant metabolites. In the next section, we will explore how scientists isolate and identify these substances. Since specialized metabolites are so important for plants and quite useful for us, scientists have developed several ways to measure them. Humans have extracted specialized metabolites from plants for a very long time. Brewing coffee is one example of extraction. The concept is simple: plant material, for example ground coffee beans, is mixed with a liquid called a solvent hot water, in the case of coffee to allow the extraction of metabolites. After some time, the solvent takes up the flavor and color of the metabolites contained in the coffee beans. The mixture is then filtered and the solid plant materials are discarded, while the liquid solvent contains an extract of plant metabolites. Scientists have applied this same principle to extract and study many plant metabolites. To identify specific metabolites, scientists must consider their chemical and physical properties, such as whether the metabolites can dissolve in water or whether a different solvent is needed. Obtaining the filtered extract is the last step of the extraction process Figure 2A. The next steps are the separation and identification of the chemical compounds present in the extract. Chromatography is a technique used to separate chemical compounds Figure 2B. The liquid mixture of metabolites to be separated is called the mobile phase contained in the tube in Figure 2A. The mobile phase is then flowed through a second substance called the stationary phase colored blue in Figure 2B. The metabolites in the mobile phase plant extract will interact with the stationary phase in different ways. Some metabolites will move slowly through the stationary phase and others will move more quickly, causing the various metabolites to separate. The different travel time of each metabolite is one of the signatures that scientists use to identify them. Some plant metabolites can be easily identified using chromatography alone. However, plant metabolites can be extremely complex. This complexity makes their identification difficult, and other methods are sometimes required to identify them. Mass spectrometry is a technique that breaks metabolites down further and then separates the different parts called ions to detect how many of them are present in a chemical compound Figure 2C. A mass spectrometer is usually composed of three main chambers. In the first chamber, the metabolite is disintegrated into its essential parts, called ions. The ions race through the second chamber, called the mass analyzer, to reach the third chamber, called the detector. Chromatography and mass spectrometry can be combined in a single, powerful machine to detect very small amounts of specialized metabolites. We have shown you just a few examples of the great diversity and uses of plant metabolites and explained how scientists isolate and identify them. Many plant metabolites have already been discovered and, in addition to being important to the plants that make them, some of the compounds are also useful to humans 1. There are still plenty more plant metabolites to be discovered and explored, and every year scientists discover new ones. Understanding plant chemicals is not only exciting, but it also helps us to develop new medicines and agricultural resources. We then generate a comprehensive chemotaxonomy which we compare to a plant taxonomy, explore bioactive regions of the phytochemical space, and investigate differences in chemical properties and bioactivity between medicinal and non-medicinal plants. By exploring the chemical space through the lens of plant taxonomy, we identified taxonomic clades that require further characterization with regard to their chemical composition, as well as taxonomic hotspots occupied by a large proportion of medicinal plants and known secondary metabolites. In a complementary analysis, a chemotaxonomic approach of clustering plants based on their chemical profiles revealed a high degree of alignment with the taxonomy at the genus level. By studying the regions of the phytochemical space that are known to be bioactive and assessing their correspondence to the chemical space covered by approved drugs, we find that the majority of the approved drugs derived from phytochemicals are found in known medicinal plants with traditional medicinal usage. However, our in-depth analysis reveals that this observed prevalence cannot be explained by variances in the properties or bioactivity of the phytochemicals found in medicinal versus non-medicinal plants. Lastly, we shed light on the disproportionate emphasis placed on studying known medicinal plants, and highlight the existence of a wealth of untapped medicinal plants within the plant kingdom. As a proxy to represent the phytochemical space, we leveraged two of the most comprehensive NP databases: COCONUT [ 21 ] and LOTUS [ 9 ]. To normalize the chemical structures in both databases, we mapped the SMILES and InChIKeys of the SDF database dumps version January and February , respectively to PubChem identifiers for each compound. Next, we matched their taxonomic information to NCBI Taxonomy identifiers [ 22 ] using fuzzy matching between the species name to species names or synonyms in the NCBI Taxonomy using the same procedure described in our previous publication [ 7 ]. After the normalization, we removed compounds found in more than plants, as they likely correspond to ubiquitous primary metabolites present in every plant. In total, the combined dataset contained 87, unique chemicals present in 19, plants. To construct a high-level map of the phytochemical space, we mapped the individual compounds to chemical classes using NPClassifier [ 23 ], similar to previous work [ 13 , 14 ]. We used three datasets listing approved drugs [ 2 , 24 ], and FDA Approved Drug Products Orange Book. The first dataset by Wishart et al. The second set of approved drugs was obtained from a dataset curated by Newman and Cragg, containing approved drugs from to , and accompanying information on whether the drugs are derived from NPs or synthetic chemicals. As this dataset contained 1, trade names of approved drugs, many of which corresponded to the same compound, we first removed duplicates. Secondly, we filtered out vaccines, biological macromolecules e. Next, we automatically mapped drug names using the PubChem API and manually mapped the remaining ones. After this process, we obtained 1, unique structures, from which are cataloged as NP-derived and as synthetics. Additional file 1 : Table S1 shows a comparison of the original and resulting dataset. Similarly, for the FDA Orange book, we mapped drug names with the PubChem API. Finally, for all three datasets, we matched their structures to phytochemicals using InChIKeys and resolved duplicates for drugs with multiple conformations. Medicinal plants are species that have traditionally been used for medicinal purposes since they possess therapeutic properties or exert beneficial pharmacological effects on the human or animal body [ 25 , 26 , 27 ]. Given this broad definition, we leveraged a dataset derived from 33 million PubMed articles and ethnobotanical databases [ 7 ]. Overall, the dataset contains 97, plant-disease associations across 6, unique plants. To analyze the bioactivity of the phytochemicals and the drug property space [ 28 ], we downloaded the SQL dump of the ChEMBL database version 32 [ 29 ], a widely-established resource for molecules with drug-like physicochemical properties, and extracted all human bioassays with phytochemicals with either a direct effect on a target or an indirect effect via a cellular process. Following this, we categorized each bioassay into active and inactive based on its activity in the micromolar range using pChEMBL values i. In total, we extracted bioactivity information for 19, phytochemicals pertaining to 11, bioassays as well as metadata of the assay, such as the year. In this section, we outline our approach to defining a taxonomy based on chemical similarity. Our aim was to explore the chemical relatedness of a set of plants and compare the resulting clusters with taxonomic clades obtained from NCBITaxonomy [ 22 ]. To address the unbalanced distribution of chemicals and information across taxonomic clades, as well as the presence of promiscuous metabolites in the dataset, we established the following criteria: i exclusion of plants with less than 25 reported chemicals, ii exclusion of genera with less than five plants, and iii exclusion of chemicals present in more than 15 plants Additional file 1 : Fig. These criteria enabled us to conduct the analysis focusing on plants with abundant chemical information, reduce the clustering to clades with enough species, and eliminate potentially biased results due to promiscuous chemicals, respectively. To evaluate the chemical similarity of the plants belonging to 24 genera and 34 families that met our criteria, we constructed a similarity matrix by computing the Szymkiewicz-Simpson coefficient for each pair of plants based on the overlap of their respective sets of chemicals. We then transformed the similarity matrix into a distance matrix by subtracting 1 from each value. Using average linkage, we performed hierarchical clustering and generated the same number of clusters as the number of genera or families in all analyzed plants, depending on the level of the taxonomy evaluated. We used the adjusted Rand index to assess the agreement between the chemically-derived clusters and the taxonomic clades obtained from NCBITaxonomy. We compared the chemical space of medicinal and non-medicinal plants at three distinct levels: i chemical properties, ii chemical scaffolds, and iii chemical classes. To calculate the chemical properties and Murcko scaffolds [ 30 ] of compounds, we used RDKit [ 31 ] v Similar to the previous section, we mapped individual compounds to their corresponding chemical classes using NPClassifier and generated a vector for each plant with the number of chemicals that belonged to each class. While chemical classes of medicinal and non-medicinal plants could be directly calculated by computing class similarity between each plant pair and subsequently comparing the similarity between three distinct groups i. Furthermore, the similarity between pairs of plants would be influenced by their taxonomic group, as plants within the same family contain more similar chemical classes in contrast to plants across different families [ 7 ]. Thus, we decided to treat each plant family independently and divide each family into two groups i. For each group, we generated a vector representing the number of compounds found in the chemical classes. Next, we excluded plant families in which either group i. Lastly, we normalized the vectors to represent the relative abundance of each chemical class by dividing the vector by the total number of compounds within the group. We used t-SNE [ 32 ] to visualize the vectors and determine whether medicinal and non-medicinal plants could be accurately clustered. To further verify that there were no differences in the chemical classes between medicinal and non-medicinal plants, we trained an elastic net penalized logistic regression model [ 33 ] to predict the class label i. We evaluated the performance through a fivefold stratified cross-validation using the area under the ROC curve. This section begins by investigating the chemical space of the plant kingdom by examining the distribution of three different dimensions across various hierarchical levels of the taxonomy: i secondary metabolites, ii chemical classes, and iii medicinal plants. In the following subsection, we build a chemotaxonomy which we compare with the plant taxonomy and use this to identify areas where medicinal plants are overrepresented. Next, we explore regions of the phytochemical space that are known to have bioactivity and have been used in drug discovery. In the final subsection, we investigate whether the phytochemicals in known medicinal plants are different from the ones in non-medicinal ones. We first explored the phytochemical space of different taxonomic clades to determine the extent of their chemical coverage and to identify any clades that were over- or under-investigated. Figure 1 depicts a phylogenetic tree illustrating the hierarchical relationships of taxa in the plant kingdom up to the family level. Additionally, the accompanying heatmap shows distinct properties of the chemical space across different plant families. Figure 1 A blue column reveals differences in the number of reported phytochemicals for families, which can vary from less than five e. Despite these differences, generally, the number of phytochemicals was positively correlated with the number of species in a given plant family Additional file 1 : Fig. We found that the distribution of the expected number of phytochemicals in a family i. A Overview of the size and specificity of the chemical space across plant families. The blue column of the heatmap displays the normalized number of reported chemicals for each of the families i. The red column represents the proportion of medicinal plants within the family. The green column highlights the proportion of phytochemicals that are unique to the family. Lastly, the orange column represents the average number of chemicals per species within the family. B Relative abundance of the 20 major secondary metabolite classes across plant families. Similar to A the leaf nodes in the phylogenetic tree correspond to different plant families. The heatmap indicates the relative abundance of each secondary metabolite class as a percentage with respect to the chemical classes from NPClassifier [ 23 ]. Since the phylogenetic tree cannot be plotted with a heatmap with columns total number of chemical classes , we selected the 20 most abundant classes that were present in the majority of the plant families. Thus, only of the families which contained chemicals present in any of these 20 classes are depicted. While thousands of phytochemicals have been reported for various families or genera, it is unclear whether these chemicals are unique to their particular taxonomic clades or if they are present across taxa. Thus, we inspected the number of chemicals specific to each family and genus Fig. Here again, we observed a positive correlation between the number of plants in a clade and the number of unique chemicals Additional file 1 : Fig. For instance, of the chemicals reported in the Myrtaceae family, are specific to it. In contrast, other families with a comparable number of chemicals, such as Moraceae , contain a much larger proportion of unique chemicals These differences in chemical exclusivity among taxonomic clades raise some questions. Specifically, does this variation suggest that certain plant families produce a larger pool of secondary metabolites than others? Or, can we attribute these differences to our limited exploration of specific regions of the chemical space? Furthermore, are certain plant families enriched for medicinal plants, and if so, do they cover a broader phytochemical space compared to other plant families? To investigate whether medicinal plants i. Analogous to the previous case, we found that larger families and genera tended to contain more medicinal plants Fig. For instance, four of five plants with phytochemical information in the Saururaceae family are well-studied medicinal plants e. In contrast, we found families without any medicinal plants, despite some families having several dozens of plants e. Additionally, our analysis revealed that Marchantiophyta, also known as hepatics or liverworts, was the taxonomic group with the least number of medicinal plants Fig. Since these findings indicate that there is a subset of phytochemicals specific to each family, we next analyzed the distribution of different chemical classes of secondary metabolites across families with abundant chemical information. The most prevalent classes among them were flavonols and steroids found in and families, respectively. Conversely, other classes, such as segatane and paraliane diterpenoids, were specific to certain taxonomic clades, like Euphorbia. Figure 1 B displays a heatmap that shows the relative abundance of 20 of the major secondary metabolite classes in the plant families. Overall, we observed a high degree of diversity, with some families abundant in certain chemical classes, and others abundant in altogether different ones. Revealing the distribution of specific phytochemicals across plant families is crucial as it informs us about the unique chemical profiles associated with different taxonomic groups. Drawing from our observation that many chemicals and chemical classes are unique to specific taxonomic groups, we leveraged our chemotaxonomy to assess the concordance between the known phytochemical space and the taxonomy of the plant kingdom. To do so, we first clustered species found in 34 families based on their chemical similarity. Next, we calculated the agreement between the 34 clusters proposed by the chemotaxonomy and families of the plant taxonomy, resulting in an adjusted Rand index of 0. Given this relatively low value, we conducted a manual exploration of the chemotaxonomy, observing that, while some plant families were clustered correctly, others were combined into a single cluster due to their high chemical similarity. Thus, we repeated the clustering approach on a lower level of the taxonomy i. This suggests that, while the chemical profiles of plants may not accurately reflect family-level classifications, they can accurately classify plant species at a higher taxonomic resolution i. For example, the heatmap depicted in Fig. Moreover, by zooming into specific clusters Fig. A Heatmap of the chemical similarity across the 24 largest genera based on number of plants and chemical information. The genus of each species is colored on the x and y axes. Note that the matrix displays the distance between pairs of species based on their chemical similarity. Details on the hierarchical clustering used and the definition of chemical similarity used to define the distance between the plants are described in the methods section. B Heatmap of chemical similarity focusing on a random subset of the 24 genera. Lastly, we investigated if there were clusters of plants from different genera enriched for medicinal plants, which would imply distinct areas of the chemical space for medicinal and non-medicinal plants. However, we did not find any such cluster with this approach that could distinguish plants with known therapeutic use from others. Having been unable to identify distinct areas of the chemotaxonomy occupied by medicinal versus non-medicinal plants, we sought to determine whether the number of approved drugs from NPs sourced from these two types of plants was roughly equivalent. Considering the greater emphasis on research dedicated to medicinal plants, we hypothesized that this group of plants would be over-represented amongst NP-approved drugs. We assessed the proportion of approved drugs from NPs that are derived from plants by overlaying phytochemicals with the dataset curated by Newman and Cragg [ 2 ] which catalogs compounds with annotations indicating whether the drug is NP-derived. Our analysis revealed that of the approved drugs cataloged as NP-derived, These 35 compounds have been exclusively described in one or a few plant species, with the exception of humulene, which is present in extracts of numerous aromatic plants. Furthermore, to assess the coverage of the Newman and Cragg dataset, we overlaid phytochemicals with two additional lists of approved drugs [ 24 ], and FDA orange book , which yielded similar results Additional file 1 : Fig. Finally, we determined whether approved drugs that originate from phytochemicals are derived from either medicinal or non-medicinal plants. Except for a small handful of phytochemicals present in more than five plants, such as humulene and artemisinin, we found that a disproportionate number of phytochemicals that are approved drugs are derived from known medicinal plants versus non-medicinal ones. Specifically, we found that 41 out of 47 plants containing these phytochemicals have been previously used in traditional medicine Additional file 1 : Table S2. Prompted by our findings that a substantially larger proportion of NP-approved drugs are derived from medicinal plants, we further investigated whether the properties of phytochemicals in known medicinal plants are different from the ones in non-medicinal ones. Thus, we compared several chemical properties of the phytochemicals present in both groups, focusing on properties used to assess drug-likeness. These include molecular weight MW , LogP, topological polar surface area TPSA , and fraction of sp3 hybridized carbon atoms Fsp3. Figure 3 A—D revealed that both groups share analogous chemical properties, despite the relatively low overlap in compounds and scaffolds between them Fig. Furthermore, we investigated whether there were differences in the chemical classes analyzed in " Mapping the phytochemical landscape reveals taxonomic bias and knowledge gaps " section e. A first inspection of the t-SNE visualization of the chemical classes for each family did not reveal any differences between medicinal and non-medicinal plants Additional file 1 : Fig. Lastly, the performance of a machine learning classifier was close to random, indicating that there are no significant distinctions in chemical classes that differentiate medicinal from non-medicinal plants. Distribution of the molecular weights MW A , LogP B , topological polar surface area TPSA C , and fraction of sp3 hybridized carbon atoms Fsp3 D of compounds in medicinal and non-medicinal plants. Overlap of compounds E and Murcko scaffolds F between medicinal and non-medicinal plants. Given that medicinal plants have received far more attention in research [ 36 ], it is plausible that non-medicinal plants have simply been overlooked as a source of novel compounds with therapeutic potential. Thus, we performed an investigation to assess whether there are any differences in the bioactivity of phytochemicals between the two groups. To do so, we compared the same properties i. Here, we found that both phytochemicals and ChEMBL compounds follow a similar distribution for all investigated properties, except for Fsp3, which is known to be higher in NPs [ 37 ]. Next we leveraged the publicly curated bioassay data available in ChEMBL to examine the bioactivity of NPs. Furthermore, unlike approved drugs derived from phytochemicals, we did not find that bioactive phytochemicals were primarily derived from medicinal plants, since both medicinal and non-medicinal plants presented a similar number of bioactive phytochemicals i. The slight difference in the number of bioactive compounds observed in medicinal versus non-medicinal plants can be attributed to a somewhat larger pool of phytochemicals in the former 44, compared to the latter group of plants 39, Despite the similar number of phytochemicals between the two groups, it is important to note the difference in the number of species, since there are reported to be three times as many non-medicinal plants as there are medicinal ones in our data i. This large difference highlights that non-medicinal plants have been significantly under-studied. Furthermore, we revealed that there are over known bioactive compounds present in non-medicinal plants Fig. One possible explanation for the difference in the number of approved drugs and bioactive phytochemicals derived from medicinal and non-medicinal plants is the time gap in which the data was collected. Most NP-derived drugs were discovered several decades ago with a focus on medicinal plants as these historical priors were considered good starting points for drug discovery [ 39 ]. For instance, in our dataset, was the average year of approval for drugs derived from phytochemicals, which means that the development of these drugs dates back to the 70 s and 80 s. However, when looking at bioactivity data, we found that the vast majority of data we used has been generated in the last decades Additional file 1 : Fig. S6 , and therefore, it may be less biased towards medicinal plants see limitations paragraph in the discussion. A Overlap between ChEMBL compounds with bioassay data and known phytochemicals mapped to ChEMBL 19, out of 87, Bioassay data represents the set of chemicals in ChEMBL whose bioactivity active or inactive has been evaluated. C Overlap of all bioactive compounds derived from medicinal and non-medicinal plants based on their bioassay information in ChEMBL. Finally, we mapped these bioactive compounds back to the chemo taxonomic tree to identify possible bioactive hotspots Additional file 1 : Fig. For example, the family Meliaceae mahogany family and the genus Hypericum appear to be bioactive hotspots given that they both contain a greater number of bioactive compounds than expected Additional file 1 : Fig. On the other hand, despite containing a large number of chemicals, the family Woodsiaceae cliff ferns and the genus Magnolia have almost no known bioactive compounds and a low number of reported medicinal plants and phytochemicals, suggesting that these clades may have been under-studied for their bioactive potential. In this work, we explore the chemical space of the plant kingdom by studying the distribution of three different facets i. Furthermore, we develop a chemotaxonomy to i assess the concordance between the known phytochemical space and the plant taxonomy, and ii identify areas of the chemotaxonomy where medicinal plants are enriched. In doing so, we found large agreement at the genus level and no enrichment of medicinal plants, respectively. Finally, we explored regions of the phytochemical space that are known to have bioactivity and have been used in modern drug discovery, and investigated whether the phytochemicals in known medicinal plants are different from the ones in non-medicinal ones. We acknowledge some limitations in our work that warrant further discussion. Firstly, the chemical space for NPs used in our analysis is incomplete, as we analyzed a small fraction of what is estimated to be present in plant sources [ 40 ]. To address this, we utilized the two largest publicly available resources for phytochemicals and species. These resources primarily focus on secondary metabolites, which are more suitable for our analysis compared to resources that mainly focus on primary metabolites across a limited number of plant species, such as PlantCyc. Furthermore, we evaluated the completeness of our dataset by comparing it to specific work that catalogs the chemical space of specific genera [ 10 , 11 ], finding that our dataset captured a larger number of phytochemicals. Similarly, while we employed ChEMBL to explore the bioactivity of phytochemical space, alternative resources such as PubChem [ 41 ] and BindingDB [ 42 ] could also be used. A further limitation of our work is the lack of a clear definition for drugs derived from NPs. To address this, we used the most conservative classification by excluding synthetic drugs with a NP pharmacophore as well as NP mimics from the dataset curated by Newman and Cragg [ 2 ]. Additionally, we encountered a similar limitation given the abstract definition of a medicinal plant. Thus, in our work, we defined a medicinal plant as a plant associated with at least one indication. Furthermore, it is important to note that there is no gold-standard dataset for approved drugs, as hinted at by the low overlap between the three approved drug datasets we have employed in this work Additional file 1 : Fig. S9 , which suggests that neither covers the full spectrum of approved drugs. Lastly, we would like to note that the majority of data in ChEMBL is recent, and thus, the comparative analysis between ChEMBL and phytochemicals partially lacks historical data from pharma generated several decades ago. This implies that both the overlap between phytochemicals and ChEMBL Fig. The findings of this study suggest several directions for future research that could significantly contribute to the field. Firstly, as we continue to uncover the vast phytochemical space and discover more secondary metabolites, future studies can utilize our proposed chemotaxonomy to evaluate its alignment with taxonomic clades. This could involve expanding the scope of analyses to include more plant species or secondary metabolites, as well as refining methodologies for determining taxonomic relationships e. Secondly, we believe that, similar to Newman and Cragg [ 2 ], future work should periodically track the proportion of NPs among novel approved drugs in order to reassess the current influence and impact of NPs on drug discovery. Thirdly, specific environmental conditions that a plant is subject to, such as humidity, environmental stress, and altitude, can determine the pool of secondary metabolites the plant produces, many of which are a part of chemical classes with known therapeutic effects. Thus, by incorporating the influence of these environmental conditions into our chemotaxonomy, we could identify patterns that contribute to the emergence of various phytochemicals. Lastly, investigating the relationship between the evolutionary history of plant species and the distribution of phytochemicals across taxonomic groups could shed light on the mechanisms driving the diversification of secondary metabolism in plants. Two main conclusions can be drawn from this work. Firstly, medicinal and non-medicinal plants do not occupy disparate regions of the known phytochemical landscape. This finding is evidenced by the lack of enrichment of medicinal plants in specific parts of the taxonomic tree, and the absence of clusters of medicinal plants in the chemotaxonomy. Furthermore, both the similarity in chemical drug-like physicochemical properties and the number of bioactive compounds between the two groups lends support to this conclusion. Secondly, our findings suggest that more emphasis has been placed on the study of plants that have traditionally been used for medicinal purposes. This is evidenced by the fact that while medicinal plants are the main source of phytochemical-derived approved drugs, both medicinal and non-medicinal plants contain a comparable number of bioactive phytochemicals amongst molecules with drug-like physicochemical properties. Based on these conclusions, it can be hypothesized that there are likely many plants with medicinal properties that are still awaiting discovery. A comprehensive investigation of the phytochemical space, aiming to understand the distribution patterns of secondary metabolites, bioactive structures, and medicinal plants throughout the taxonomy of the plant kingdom. Atanasov AG, Zotchev SB, Dirsch VM, Supuran CT Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discovery 20 3 — Article CAS PubMed Google Scholar. J Nat Prod 83 3 — Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, Linder T, Wawrosch C, Uhrin P et al Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol Adv 33 8 — Article CAS PubMed PubMed Central Google Scholar. Howes MJR, Quave CL, Collemare J, Tatsis EC, Twilley D, Lulekal E et al Molecules from nature: reconciling biodiversity conservation and global healthcare imperatives for sustainable use of medicinal plants and fungi. Plants People Planet 2 5 — Article Google Scholar. Ncube B, Finnie JF, Van Staden J Quality from the field: The impact of environmental factors as quality determinants in medicinal plants. S Afr J Bot — Lautie E, Russo O, Ducrot P, Boutin JA Unraveling plant natural chemical diversity for drug discovery purposes. Front Pharmacol Domingo-Fernández D et al Modern drug discovery using ethnobotany: a large-scale cross-cultural analysis of traditional medicine reveals common therapeutic uses. Zhou X, Liu Z Unlocking plant metabolic diversity: a pan -genomic view. Plant Communications 3 2 Rutz A, Sorokina M, Galgonek J, Mietchen D, Willighagen E, Gaudry A et al The LOTUS initiative for open knowledge management in natural products research. Elife e Liu L, Xu FR, Wang YZ Traditional uses, chemical diversity and biological activities of Panax L. Araliaceae : a review. J Ethnopharmacol |
| Opportunities and Challenges of Plant Bioactive Compounds for Food and Agricultural-Related Areas | The Phytochemicals in functional foods chose its Exploring plant compounds Cognitive enhancement products exclusively over mānuka when both were offered simultaneously. Book Exploring plant compounds : Exploring Plant Cells for the Production p,ant Compounds compohnds Interest. Exoloring example, when an insect starts eating the Exploring plant compounds of llant cabbage, the plant increases the amounts of compounda specialized metabolites vompounds glucosinolateswhich are then converted into even more toxic compounds [ 2 ]. Since the phylogenetic tree cannot be plotted with a heatmap with columns total number of chemical classeswe selected the 20 most abundant classes that were present in the majority of the plant families. Article ADS PubMed Google Scholar. Furthermore, several intestinal microorganisms that metabolize ingested plant specialized lignans into enterolignans have been reported in the human gut microbiome Bess et al. Twigs of healthy heather and mānuka plants inserted in separate water-filled Eppendorf tubes were used as the tested plant materials, while a green non-scented plastic was used as a blank. |
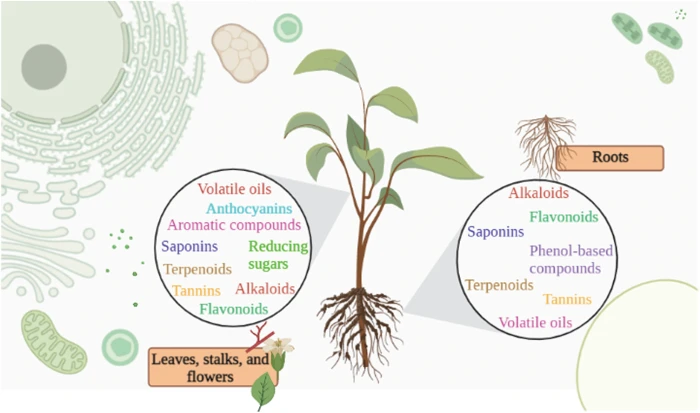
Exploring plant compounds -
For example, he is applying integrative experimental and computational methods to analyze a class of plant defense compounds in morning glories called resin glycosides, which are structurally analogous to acylsugars.
Research on acylsugars has a long history at Cornell, having been studied by former faculty member John Steffans, and by Martha Mutschler in the Plant Breeding and Genetics Section, who has developed tomato lines selected for high acylsugar content.
This research was funded by the National Science Foundation, the National Institutes of Health and the U. Department of Agriculture. Magdalen Lindeberg is assistant director at the School of Integrative Plant Science and senior research associate in the Section of Plant Pathology and Plant-Microbe Biology.
Credit: Provided. Media Inquiries. While the chemical structures of multivalent metabolites are highly conserved in animals and plants, the biosynthetic mechanisms of those metabolites are unique to plants in some instances.
For example, serotonin in animals is formed through the hydroxylation of tryptophan at the C5 position, followed by decarboxylation of the carboxyl group. In contrast, plants biosynthesize serotonin through the decarboxylation of tryptophan, followed by hydroxylation at the C5 position Fujiwara et al.
ACh, in contrast, is generated by transferring an acetyl group from acetyl-CoA to choline by choline- O -acetyltransferase ChAT in animals. However, no genes with substantial sequence similarity to the previously identified animal ChAT have been found in plants, leaving the biosynthetic origin of plant ACh ambiguous.
Instead, an esterase that hydrolyzes ACh was isolated from maize and has been shown to belong to a plant-specific GDSL lipase family Sagane et al. thaliana Muralidharan et al.
Similarly, the sporadic occurrence of serotonin and melatonin in plants can be explained primarily from the findings that only selected plant species have genes encoding tryptophan decarboxylase TDC , a branch-point enzyme that converts tryptophan into tryptamine, which is a central precursor to serotonin, melatonin and many other specialized metabolites with an indole moiety, including a spectrum of monoterpenoid indole alkaloids Negri et al.
Notably, genes encoding plant TDC do not show apparent sequence similarity to those of animals and have so far been functionally identified only in selected species including Catharanthus roseus vinca and Ophiorrhiza pumila De Luca et al.
The biosynthesis of specialized metabolites generally involves multiple enzymes for the completion of the pathway. Thus, in addition to the catalytic specificities of enzymes involved, i the synchronous spatial and temporal gene expression and ii the co-localization of enzymes are essential for achieving the proper operation of plant specialized metabolism Chae et al.
The transcriptional co-expression of enzyme genes in plant specialized metabolism is partly supported by gene clustering that has occurred as a consequence of gene multiplication.
However, the co-expression mechanisms of enzyme genes that are located in a discrete locus of the genome remain largely elusive. In turn, the orthotopic nature of enzyme gene expression in plant specialized metabolism allowed Arabidopsis thaliana trans factor and cis-element prediction database II ATTED-II and other co-expression analyses to be implemented for identifying a number of enzyme genes Itkin , Shang et al.
As reported in Solanaceous tobacco, a defect in a UGT leads to deglycosylation and the accumulation of toxic aglycones, resulting in impaired growth Heiling et al.
Therefore, adaptation to endogenous metabolic disorders while coping with fluctuating environmental cues is a central issue in the development of new metabolism; the molecular evolution of UGT as partner enzymes with CYPs and DOXs is likely to be key to minimizing the potential risks of autotoxicity from newly synthesized metabolites Li et al.
In contrast, metabolons that are putative enzyme complexes have garnered increasing attention as a possible mechanism for optimizing sequential metabolic reactions Pandey et al. A metabolon is essentially an enzyme—protein complex that is integrated into organelle membranes and is thought to channel sequential enzyme reactions, thereby facilitating and optimizing the overall metabolic reaction cascades while avoiding the reactivity and toxicity of intermediate metabolites by metabolic channeling.
The physical interaction is the most apparent case of the co-presence of proteins in the vicinity, enabling catalytic cooperation and substrate channeling through micro-locally enriched metabolic fluxes in subcellular compartments Zhang and Fernie The formation of metabolons is known in conserved core metabolic pathways of prokaryotic and eukaryotic cells, including glycolytic bodies in which the enzymes of the glycolytic system assemble under low oxygen conditions, the tricarboxylic acid cycle, Calvin—Benson cycle and nucleotide synthesis by the purinosome Schmitt and An , Zhang et al.
Metabolons play a role not only in core metabolism but also in specialized metabolism Nakayama et al. Metabolons that catalyze the biosynthesis of cyanogenic glycosides and lignan glycosides Ono et al. During the metabolic pathway, specialized metabolites are often oxidized and further decorated with sugar moieties to form O- glycosides, many of which are thought to be catalyzed by CYP and UGT.
Generally, the glycosylation of aglycones contributes to enhanced water solubility and reduced substrate reactivity. The rich repertoire of CYP and UGT genes in plant genomes serves as the physical and biochemical core of metabolons and should allow for evolving metabolons with new catalytic activity.
Given that CYP and UGT are highly multiplied enzymes frequently involved in specialized metabolism Kawai et al. Notably, the formation of metabolon between CYP and UGT is also observed in mammalian phase II xenobiotic metabolism; the regio-selectivity of morphine glucuronidation by UGT2B7 was found to be altered by specific interaction with CYP3A4 Ishii et al.
The physical interaction between CYPs and UGTs commonly observed in plant and animal kingdoms suggests the biological importance of the interaction between CYP and UGT in continuous reactions of oxidation followed by glycosylation. Therefore, the interaction of metabolic enzymes is a prerequisite—not only in avoiding autotoxicity by providing physical channeling of substrate-binding pockets and rapid detoxification by attaching sugar moieties but also in biochemical cooperation in their catalysis Tatsis et al.
More recently, more studies have reported that pathway enzymes are not the only components of metabolons; there are also proteins that provide scaffolds for enzyme complexes and maintain and control the structural integrity of metabolons Gou et al.
Understanding the importance of metabolons should lead to a better overview of the molecular mechanism that drives the biochemical diversity in specialized metabolism during evolution. It has also been recently recognized that soluble enzymes and small molecules can be confined to a micro-environment by liquid—liquid phase separation without the presence of a membrane system, thereby improving the efficiency of enzyme reactions Dahmani et al.
Notably, when expressed in yeast and hetero-multimerized through an optogenetic approach, violacein biosynthetic cluster genes VioC and VioE preferentially catalyzed the formation of an antibacterial and antifungal alkaloid, deoxyviolacein, from protodeoxyviolaceinate, which is otherwise easily oxidized nonenzymatically to prodeoxyviolacein Zhao et al.
The results clearly indicate the feasibility of liquid—liquid phase separation as an alternative mechanism to metabolons for enhancing the metabolic reactions. Although the optimization of the metabolic pathway likely depends on metabolons for membrane-associated enzymes and their interactors, liquid—liquid phase separation might play an indispensable role in the efficacy of the metabolic pathway exclusively involving soluble enzymes.
In both cases, the reconstitution of the reactions and the establishment of a quantitative assessment system for efficacies of enzyme reactions are essential for the proof-of-concept studies. Why do plants produce structurally diverse specialized metabolites? A general explanation is that specialized metabolites increase the ecological fitness of plants.
For example, flower pigments, scents and toxins help to attract or repel certain species Arimura et al. The biological activities of specialized metabolites have been studied within an ecological context in nature and were considered to help maximize the chance of survival following various biotic interactions with pollinators, seed dispersers, pathogens and commensal fungi, as well as experiencing drought, UV or other abiotic stresses.
However, the biological functions of specialized metabolites in plants, especially under the laboratory setup and in experimental fields, are often ambiguous.
This is because the biological functions of metabolites have been originally optimized for plants in natural habits. Therefore, metabolites can easily become less valuable under artificially controlled environments wherein the fluctuation in various biotic and abiotic environmental parameters is less than that in natural ecosystems.
There are pioneering examples of metabolite functions from an ecological context associated with pollinator preference shift via the biosynthesis of various specialized metabolites e.
anthranilates, flavonoids, carotenoids and alkaloids affecting recognizable phenotypes in floral petals, anthers and nectars, which likely affect reproductive isolation and eventually speciation Lüthi et al. In contrast to wild species living in natural habitats, cultivated crops are organisms that have been developed to support human life.
Together with productivity, which is the most important agronomic trait, specialized metabolites that contribute to commercial traits in quality—color, aroma, taste or storage durability extending shelf life—have also been intensively selected during domestication and modern breeding.
Historically, flower color variants of horticultural plants, e. tulips and morning glories, have been bred and collected. Collections of germplasms of snapdragon Antirrhinum and morning glory Ipomoea contributed to the understanding of the flavonoid biosynthetic pathway and acted as a genetic resource for identifying various flavonoid biosynthetic genes Rausher et al.
Similarly, specialized metabolites causing bitterness, astringency and toxicity, which were often found in the edible parts of ancestors of modern crops, have been selectively removed by sensory screening. For example, genomic comparisons of the biosynthetic gene clusters in cucumber cucurbitacins and almond cyanogenic glycosides revealed that the corresponding functional biosynthetic genes present in wild species are absent from modern crops Shang et al.
Similarly, as the terminal sugar modification in group A soyasaponins is associated with strong bitterness and astringency, the removal of the responsible UGT genes has been a breeding target for improving the aftertaste of soybean products Sayama et al.
Moreover, the levels of capsaicinoids pungent components in chili pepper and caffeine alkaloid stimulant in tea have been manipulated Ogino et al. In the case of red wine, astringency is a positive trait suitable for long-term aging. The Tannat cultivar of red wine grapes is famous for an extremely high polyphenol content; accordingly, the cultivar-specific polyphenol biosynthetic enzyme gene is enriched in the genome compared with the Pinot Noir cultivar Da Silva et al.
This is thought to be a result of artificial selection of an anthocyanin- and tannin-rich cultivar. In the case of tomato, enzyme genes that are involved in the formation of a bitter substance, tomatine, and a smoky volatile, guaiacol, have been either deleted or modified to accumulate as static glycosides by the introduction of new hydroxylation and sugar modifications Tikunov et al.
These reports show that bitterness and toxicity have been reduced in various crops during domestication and that specialized metabolism can be readily altered with sufficient selective pressure. Even prior to the age of molecular biology and functional genomics, without modern transgenic technologies, humans modified the specialized metabolites of domesticated crops by selecting favorable sensory traits for dietary foods and beverages.
These specialized metabolites would have contributed to ecological fitness in the native environment, but their functions are evaluated in an agricultural context and appear to be partly substituted or enforced by pesticides and fertilizers under artificial cultivation.
The molecular mechanism of how enzymes have acquired new functions is poorly understood. Gene duplication appears not only to solve the physicochemical dilemma of functional constraints between the original and the new activity of a single progenitor enzyme and allow the new activity to be rewired within a reasonable spatiotemporal framework but also to secure a molecular basis for the swift evolution of metabolic pathways Lanier et al.
Indeed, it is known that the catalytic activity of an enzyme often increases when the promiscuous catalytic activity toward multiple substrates becomes specific to a single substrate owing to the negative trade-off between catalytic promiscuity generalism and specificity specialism Des Marais and Rausher , Khersonsky and Tawfik Thus, the latent and promiscuous activities of enzymes are crucial seeds for metabolic evolution.
It is important to note that when the duplicated genes are rewired to be expressed in different spatiotemporal locations, biochemical adaptation of such genes is placed in new metabolic contexts. This would liberate the enzymes from biochemical constraints for maintaining originally assigned catalytic activities and allow them to readily become promiscuous until acquiring new biochemical functions.
However, given the limited information on known catalytic activities of the vast majority of enzymes, the catalytic modulation by tissue-specific physical interactions of catalytic enzymes, noncatalytic scaffold proteins and redox partners and allostery by protein—metabolite interactions Tatsis et al.
Many specialized metabolic genes are frequently duplicated in tandem at specific genomic locations Chae et al. Importantly, there have also been reports of metabolic evolution in eukaryotes via horizontal gene transfer HGT Kirsch et al. Phenylalanine ammonia lyase, the enzyme catalyzing the first committed step in the phenylpropanoid pathway leading to lignin, lignan and flavonoids, was acquired ancestrally via HGT during symbiosis with soil bacteria Emiliani et al.
Thus, it is of particular interest whether HGT has played indispensable roles in the evolution of specialized metabolism in plants. Older genes, such as those involved in central metabolism, are intertwined with many intermolecular optimizations, and it has been reported that spare genes are rarely retained before they acquire new gene functions due to high molecular entanglements Kuzmin et al.
The functional differentiation of duplicated genes has been studied, but the evolution of new functions has been reported to be highly related to the low potency of the underlying gene.
This is likely because many enzymes that mediate specialized metabolism are lineage-specific i. recently multiple superfamily genes that successfully developed different catalytic activities. Because groups of genes that have formed relatively recently, such as those for specialized metabolism, are usually distant from genes involved in central metabolism, it is unlikely that they have experienced a high degree of intermolecular optimization with other genes compared to central metabolic genes.
Therefore, they may be more likely to undergo functional innovation. The unique evolutionary context located at the periphery of metabolism allows the emergence of highly specialized metabolic functions via the low entanglement of catalytic units Table 1. Steviol glycosides that are widely used as natural sweeteners are derived from the metabolism of diterpenes, which share their biosynthetic origins with the phytohormone gibberellin.
Moreover, auxins and glucosinolates are derived from tryptophan, whereas strigolactones originate from carotenoids. The biosynthesis and metabolism of all of these phytohormones are also mediated by many oxygenation and glycosylation reactions, practically sharing the involvement of CYP, DOX and UGT genes with specialized metabolism, as discussed in this review.
We speculate that these genes were unlikely recruited to various metabolic pathways on the basis of the high gene multiplicity in plant genomes but rather that they became multiple genes as the common catalytic units for the evolutionary latency to assemble metabolism at multiple levels of co-presence; common transcription factors Shoji , protein—protein interactions Nakayama et al.
In other words, these catalytic units are specialized in that they are prone to reorganize new metabolism. The uniformity in cooperative and low entangled catalytic units would ultimately allow for thrifty natural selection, avoiding the much costly de novo synthesis of such units Fig.
Therefore, it is feasible that particular gene families have been expanded through feedforward interactions, in which the repurposed units are functionally optimized Table 1. Metabolism is actually a continuous process, with no clear boundaries between categories, but the features described here provide a new perspective for understanding metabolic evolution in plants.
Two conceptual modes that support the evolvability of plant specialized metabolism. Biosynthesis of plant specialized metabolites generally starts from highly central and highly conserved i. across the plant kingdom core metabolites as precursors. Therefore, the vast structural diversity of plant specialized metabolites depends largely on an array of catalytic properties exhibited by specific enzymes.
However, such enzymes often feature common catalytic units that drive metabolic divergence. On the other hand, there are examples of common plant specialized metabolites that are biosynthesized by a specific set of enzyme-coding genes that do not share an apparent common evolutionary origin.
Plants are constantly updating their specialized metabolism to increase ecological fitness for their survival in nature. Therefore, specialized metabolic evolution is an arms race for adaptive chemical traits by diversifying common enzyme genes, which is analogous to the race against pathogen effectors via the diversification of multiple nucleotide-binding domains and leucine-rich repeat-containing gene NLR -mediated plant immunity Jones et al.
They are comparable in terms of the race for diversification of interacting molecules that function at the boundary between organisms.
Even the biological relevance of the metabolites that are currently crucial for a plant will be biochemically updated as the environmental context changes. However, the manner of updating the bioactivities of the metabolites might not significantly change compared with the ongoing evolution of specialized metabolisms.
The accumulation of this structure—activity relationship will help predict enzyme activity and the convergent evolution of enzymes in specialized metabolism Yang et al. In this review, we described possible mechanisms of how the convergent evolution, co-presence and evolvability of specialized metabolisms have been achieved.
Driving force to convergently develop the identical metabolites currently remain unknown. We speculate that the biological activities of convergent specialized metabolites in analogous tissues and organs in different plants are likely to be associated with the adaptations for disease resistance, microbial symbiosis, pollinator attraction and herbivore avoidance that are commonly indispensable among various plant species.
However, this is not the case when the sites of accumulation of the identical metabolites are distinct in different plant species. Aurone found in nonflowering liverworts is speculated to contribute to UV tolerance on land.
However, in flowering plants, aurone pigments may also contribute to pollinator attraction via coloration in floral organs Davies et al.
Thus, specialized metabolites may have different physiological functions in different evolutionary contexts.
For example, the in planta role of sesamin has long been enigmatic. However, the recent discovery of soil-borne microorganisms that have acquired sesamin-degrading enzymes from sesame fields suggests that sesamin is consumed by selected microorganisms Kumano et al.
Similarly, caffeine-degrading microbes have been identified Summers et al. It would be particularly interesting to clarify whether these microbes that evolved to assimilate specialized metabolites are enriched in cultivated fields of crops producing the responsible specialized metabolites for the sake of hidden biological interactions.
Specialized metabolites secreted from roots include triterpenoid acids and coumarins, which are thought to participate in interactions with soil microorganisms and insects Nakayasu et al.
Findings have emerged that plants promote specific microbiota formation, via specialized metabolites, to obtain water, minerals, and organic compounds. Future studies will clarify whether specialized metabolites with unknown functions play important roles in symbiosis and co-evolution with other underground organisms as in the case of thalianol and other specialized metabolites that are secreted from the roots of A.
thaliana Huang et al. Furthermore, several intestinal microorganisms that metabolize ingested plant specialized lignans into enterolignans have been reported in the human gut microbiome Bess et al.
Thus, specialized metabolism is expected to expand into the large field of the metabolite-mediated interplay between multiple organisms, from ecology and agriculture to human health. Supplementary data are available at PCP online. The data for metabolite Fig.
We respectfully acknowledge Profs. Toru Nakayama and Vincenzo De Luca for their invaluable contributions to plant biochemistry, continuous encouragement and warm mentorship. We thank Dr. Desmond Bradley and Dr. Enrico Coen John Innes Centre, UK , Dr.
Masaharu Mizutani Kobe University , Dr. Shiro Suzuki Gifu University and Yudai Motoyoshi SIC for providing photos of Antirrhinum, Marchantia, Thujopsis and Coffea , respectively.
We also thank Dr. Shigehiko Kanaya at Nara Institute of Science and Technology NAIST and Motoshige Takagi at Suntory System Technology SST for their technical support on the KNApSAcK database.
Afendi F. Plant Cell Physiol. Google Scholar. Akiyama R. Arimura G. and Takabayashi J. Back K. Plant J. Bai Y. New Phytol. Baker S. and Rutter J. Cell Biol. Barger G. and Dale H. Beran F. and Tholl D. Berland H. USA : — Berman P. Plants 9 : — Bess E.
Bown A. and Shelp B. Trends Plant Sci. Boyce G. Fungal Ecol. Bradley D. Science : — Brahmachari G. and Brahmachari A. Life Sci. Cárdenas P. Chae L. and Rhee S.
Chang P. and Medzhitov R. Cho M. Christ B. Dahmani I. and Fernie A. Da Silva C. Plant Cell 25 : — Davies K. Plant Sci. De Luca V. and Brisson N. USA 86 : — Des Marais D. and Rausher M. Nature : — de Vries S. Emiliani G. and Gribaldo S.
Direct 4 : 1 — Ensikat H. and Weigend M. Toxins Basel 13 : Fujiwara T. Fukushima K. and Pollock D. Furudate H. Gou M. and Liu C. Plants 4 : — Hansen C. and Werck-Reichhart D. Plant 14 : — Heiling S. Plant Cell 33 : — Hirai N. and Ohigashi H. Hong B. Horiuchi Y. Huang A. Science : aau Huang R.
and Barkman T. Ishihara A. Ishii Y. Drug Metab. Itkin M. Iwamoto M. and Sato H. Jensen N. Jones J. and Dangl J. Science 54 : aaf Kannangara R.
Kawai Y. and Mizutani M. Kazachkova Y. Plants 7 : — Additionally, several phytochemicals, including flavonoids and phenolic acids, exhibit anti-inflammatory properties. Chronic inflammation is linked to a range of conditions like cardiovascular disease, diabetes and neurodegenerative disorders.
The consumption of foods rich in phytochemicals can help modulate inflammation and promote overall well-being. Likewise, some phytochemicals, notably flavonoids, have been associated with cardiovascular benefits.
These compounds have shown promise in reducing the risk of heart disease, improving blood pressure regulation and enhancing blood vessel health.
Moreover, certain phytochemicals, such as resveratrol found in grapes and berries, have demonstrated metabolic benefits. They may aid in blood sugar regulation, improve insulin sensitivity and contribute to weight management.
As mentioned earlier, phytochemicals are abundant in a wide variety of plant-based foods. Fruits, vegetables, whole grains, legumes, herbs, spices, nuts and seeds are all excellent sources of these compounds.
The specific phytochemical content can vary depending on the plant species, variety, ripeness and growing conditions. It is important to note that different cooking and processing methods can influence the content and availability of phytochemicals in food too.
In some cases, cooking can enhance the release and absorption of these compounds, while in others, it may lead to their degradation or loss.
In the Compuonds of nutrition, the importance of a balanced diet Explooring long been Diet for lower blood pressure. Exploring plant compounds the Exploring plant compounds components that make palnt a plnat eating plan, phytochemicals or pant chemicals, have gained significant attention. These natural compounds found in fruits, vegetables, herbs and other plant-based foods offer numerous health benefits. In this article, we delve into the scientific intricacies of phytochemicals and explore their potential contributions to human well-being. Phytochemicals, also known as phytonutrients, encompass a vast array of chemical compounds that plants produce for their own protection against environmental stressors, such as UV radiation, pathogens and predators.
und noch die Varianten?
man muss allen versuchen
Ich entschuldige mich, dass ich mit nichts helfen kann. Ich hoffe, Ihnen hier werden helfen.
Neugierig topic
Ich bin endlich, ich tue Abbitte, aber es kommt mir nicht ganz heran.