Video
Lipid (Fat) Metabolism Overview, AnimationSpeedy lipid breakdown -
Whereas the initial generation of lipid droplets is usually protective, long-term accumulation and uncontrolled enlargement of lipid droplets in chronic disease can exacerbate disease progression. It is thus necessary that the unique protein and lipid fingerprint of physiological and pathological lipid droplets is dissected to enable greater insights into their transition from being beneficial to being detrimental in different disease settings.
How lipid droplets interact with other organelles in health and disease states, and whether such contacts contribute to disease progression, also remain to be determined.
Technological breakthroughs providing sensitive and specific methods for accurate detection and visualization of lipids in their natural environments will no doubt prove pivotal in facilitating these advances in our understanding. In turn, the development of lipid droplet-based therapeutic strategies will be greatly enhanced by improving our understanding of the biogenesis and function of lipid droplets.
Altman, R. Die Elementarorganismen und Ihre Beziehungen zu den Zellen [German] Viet, Wilson, E. The Cell in Development and Inheritance Macmillan, Greenberg, A. et al. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets.
Article CAS PubMed Google Scholar. Huang, A. Oil bodies and oleosins in seeds. Plant Physiol. Plant Mol. Article CAS Google Scholar.
Walther, T. Lipid droplet biogenesis. Cell Dev. Article CAS PubMed PubMed Central Google Scholar. Henne, W. The assembly of lipid droplets and their roles in challenged cells. EMBO J. Article PubMed PubMed Central Google Scholar.
Olzmann, J. Dynamics and functions of lipid droplets. Cell Biol. Gao, M. The biogenesis of lipid droplets: lipids take center stage. Lipid Res. Wanner, G. The ontogeny of lipid bodies spherosomes in plant cells: ultrastructural evidence.
Planta , — Murphy, D. Mechanisms of lipid-body formation. Trends Biochem. Zweytick, D. Intracellular lipid particles of eukaryotic cells.
Acta , — Robenek, M. Lipids partition caveolin-1 from ER membranes into lipid droplets: updating the model of lipid droplet biogenesis. FASEB J. Wältermann, M.
Mechanism of lipid-body formation in prokaryotes: how bacteria fatten up. Article PubMed Google Scholar. Andersson, L.
PLD1 and ERK2 regulate cytosolic lipid droplet formation. Cell Sci. Wolins, N. A proposed model of fat packaging by exchangeable lipid droplet proteins. FEBS Lett. Ploegh, H. A lipid-based model for the creation of an escape hatch from the endoplasmic reticulum.
Nature , — Ohsaki, Y. Biogenesis of cytoplasmic lipid droplets: from the lipid ester globule in the membrane to the visible structure.
Skinner, J. The life of lipid droplets. Choudhary, V. The topology of the triacylglycerol synthesizing enzyme Lro1 indicates that neutral lipids can be produced within the luminal compartment of the endoplasmatic reticulum: implications for the biogenesis of lipid droplets.
Brasaemle, D. Packaging of fat: an evolving model of lipid droplet assembly and expansion. Pol, A. Review: biogenesis of the multifunctional lipid droplet: lipids, proteins, and sites. Thiam, A. Lipid droplet nucleation. Trends Cell Biol.
Cases, S. Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Natl Acad. USA 95 , — Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members.
Lardizabal, K. DGAT2 is a new diacylglycerol acyltransferase gene family. Chang, C. Molecular cloning and functional expression of human acyl-coenzyme A: cholesterol acyltransferase cDNA in mutant Chinese hamster ovary cells.
Anderson, R. Identification of a form of acyl-CoA:cholesterol acyltransferase specific to liver and intestine in nonhuman primates. ACAT-2, a second mammalian acyl-CoA:cholesterol acyltransferase. Its cloning, expression, and characterization. Wang, L. Structure and mechanism of human diacylglycerol O-acyltransferase 1.
Sui, X. Structure and catalytic mechanism of a human triacylglycerol-synthesis enzyme. Guan, C. Structural insights into the inhibition mechanism of human sterol O-acyltransferase 1 by a competitive inhibitor. Long, T. Structure of nevanimibe-bound tetrameric human ACAT1.
Molecular structures of human ACAT2 disclose mechanism for selective inhibition. Structure 29 , — e4 Oelkers, P. A lecithin cholesterol acyltransferase-like gene mediates diacylglycerol esterification in yeast.
The DGA1 gene determines a second triglyceride synthetic pathway in yeast. Yang, H. Sterol esterification in yeast: a two-gene process. Science , — Sandager, L.
Storage lipid synthesis is non-essential in yeast. Sorger, D. A yeast strain lacking lipid particles bears a defect in ergosterol formation. Harris, C. DGAT enzymes are required for triacylglycerol synthesis and lipid droplets in adipocytes.
Petschnigg, J. Good fat, essential cellular requirements for triacylglycerol synthesis to maintain membrane homeostasis in yeast.
Guo, Y. Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Sturley, S. Lipid droplet formation on opposing sides of the endoplasmic reticulum. Hamilton, J. Solubilization and localization of triolein in phosphatidylcholine bilayers: a 13C NMR study.
USA 78 , — Khandelia, H. Triglyceride blisters in lipid bilayers: implications for lipid droplet biogenesis and the mobile lipid signal in cancer cell membranes. PLoS ONE 5 , e Duelund, L. Composition, structure and properties of POPC-triolein mixtures. Evidence of triglyceride domains in phospholipid bilayers.
The physics of lipid droplet nucleation, growth and budding. A conserved family of proteins facilitates nascent lipid droplet budding from the ER. Zoni, V. Pre-existing bilayer stresses modulate triglyceride accumulation in the ER versus lipid droplets.
eLife 10 , e ER membrane phospholipids and surface tension control cellular lipid droplet formation. Cell 41 , — e7 Architecture of lipid droplets in endoplasmic reticulum is determined by phospholipid intrinsic curvature. CB 28 , — e9 Santinho, A.
Membrane curvature catalyzes lipid droplet assembly. CB 30 , — e6 Adeyo, O. The yeast lipin orthologue Pah1p is important for biogenesis of lipid droplets. Fei, W. PLoS Genet. Szymanski, K. The lipodystrophy protein seipin is found at endoplasmic reticulum lipid droplet junctions and is important for droplet morphology.
USA , — Fld1p, a functional homologue of human seipin, regulates the size of lipid droplets in yeast. Klug, Y. Arlt, H. Seipin forms a flexible cage at lipid droplet formation sites. Salo, V. Seipin facilitates triglyceride flow to lipid droplet and counteracts droplet ripening via endoplasmic reticulum contact.
Cell 50 , — Chung, J. LDAF1 and seipin form a lipid droplet assembly complex. Cell 51 , — Seipin and Nem1 establish discrete ER subdomains to initiate yeast lipid droplet biogenesis. Wang, S. Seipin and the membrane-shaping protein Pex30 cooperate in organelle budding from the endoplasmic reticulum.
Joshi, A. Lipid droplet and peroxisome biogenesis occur at the same ER subdomains. Multiple C2 domain-containing transmembrane proteins promote lipid droplet biogenesis and growth at specialized endoplasmic reticulum subdomains.
Cell 32 , — Chen, F. FIT2 organizes lipid droplet biogenesis with ER tubule-forming proteins and septins. Jacquier, N. Lipid droplets are functionally connected to the endoplasmic reticulum in Saccharomyces cerevisiae. Grippa, A. Valm, A. Applying systems-level spectral imaging and analysis to reveal the organelle interactome.
The biophysics and cell biology of lipid droplets. Deslandes, F. Lipid droplets can spontaneously bud off from a symmetric bilayer. Hegaard, F. Lens nucleation and droplet budding in a membrane model for lipid droplet biogenesis. Langmuir 38 , — Chorlay, A.
An asymmetry in monolayer tension regulates lipid droplet budding direction. Membrane asymmetry imposes directionality on lipid droplet emergence from the ER.
Cell 50 , 25— To bud or not to bud: a perspective on molecular simulations of lipid droplet budding. Hayes, M. Cell 5 , 88— Becuwe, M. FIT2 is an acyl-coenzyme A diphosphatase crucial for endoplasmic reticulum homeostasis. Gong, J. Fsp27 promotes lipid droplet growth by lipid exchange and transfer at lipid droplet contact sites.
Krahmer, N. Phosphatidylcholine synthesis for lipid droplet expansion is mediated by localized activation of CTP: phosphocholine cytidylyltransferase. Cell Metab. Wilfling, F. Triacylglycerol synthesis enzymes mediate lipid droplet growth by relocalizing from the ER to lipid droplets.
Cell 24 , — Prévost, C. Mechanism and determinants of amphipathic helix-containing protein targeting to lipid droplets. Cell 44 , 73— Olarte, M.
The CYTOLD and ERTOLD pathways for lipid droplet-protein targeting. Song, J. Identification of two pathways mediating protein targeting from ER to lipid droplets. Du, X. ORP5 localizes to ER—lipid droplet contacts and regulates the level of PI 4 P on lipid droplets.
Leduc, E. An electron microscope study of intranuclear inclusions in mouse liver and hepatoma. Thoenes, W. Fett im nucleolus [German].
Karasaki, S. The fine structure of proliferating cells in preneoplastic rat livers during azo-dye carcinogenesis. Passage of cytoplasmic lipid into interphase nuclei in preneoplastic rat liver. Taurino, M. SEIPIN proteins mediate lipid droplet biogenesis to promote pollen transmission and reduce seed dormancy.
Romanauska, A. The inner nuclear membrane is a metabolically active territory that generates nuclear lipid droplets. Cell , — e18 Reprogrammed lipid metabolism protects inner nuclear membrane against unsaturated fat.
Cell 56 , — e3 Lee, J. Lipid-associated PML structures assemble nuclear lipid droplets containing CCTα and Lipin1. Life Sci. Alliance 3 , e Morales, A. Loss of ephrin B2 receptor EPHB2 sets lipid rheostat by regulating proteins DGAT1 and ATGL inducing lipid droplet storage in prostate cancer cells.
Mosquera, J. Nuclear lipid droplets and nuclear damage in Caenorhabditis elegans. Sołtysik, K. Nuclear lipid droplets derive from a lipoprotein precursor and regulate phosphatidylcholine synthesis. Nuclear lipid droplets form in the inner nuclear membrane in a seipin-independent manner.
Pagac, M. SEIPIN regulates lipid droplet expansion and adipocyte development by modulating the activity of glycerolphosphate acyltransferase. Cell Rep. Yan, R. Human SEIPIN binds anionic phospholipids. Cell 47 , — Cartwright, B. Seipin performs dissectible functions in promoting lipid droplet biogenesis and regulating droplet morphology.
Cell 26 , — Wolinski, H. Seipin is involved in the regulation of phosphatidic acid metabolism at a subdomain of the nuclear envelope in yeast.
Duo in a mystical realm — nuclear lipid droplets and the inner nuclear membrane. Contact 2 , Article Google Scholar. Ginsberg, H. The ever-expanding role of degradation in the regulation of apolipoprotein B metabolism. Raabe, M. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice.
PML isoform II plays a critical role in nuclear lipid droplet formation. Chen, N. Fibroblasts lacking nuclear lamins do not have nuclear blebs or protrusions but nevertheless have frequent nuclear membrane ruptures. Mishra, S. Mature lipid droplets are accessible to ER luminal proteins. CAS PubMed Google Scholar.
Tiwari, S. Intracellular trafficking and secretion of VLDL. Wang, H. Proteomic and lipid characterization of apolipoprotein B-free luminal lipid droplets from mouse liver microsomes. Kim, S. Computational studies of lipid droplets. B , — Ladinsky, M.
Electron tomography revels that milk lipids originate from endoplasmic reticulum domains with novel structural features. Mammary Gland. Neoplasia 24 , — Monks, J. Organellar contacts of milk lipid droplets. Zhang, Q. Schizosaccharomyces pombe cells deficient in triacylglycerols synthesis undergo apoptosis upon entry into the stationary phase.
Conditions of endoplasmic reticulum stress stimulate lipid droplet formation in Saccharomyces cerevisiae. Welte, M. Lipid droplet functions beyond energy storage. Article CAS PubMed Central Google Scholar. Lee, Y. Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-beta-cell relationships.
USA 91 , — Listenberger, L. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Piccolis, M. Probing the global cellular responses to lipotoxicity caused by saturated fatty acids. Cell 74 , 32— e8 Song, Y.
Cholesterol-induced toxicity: an integrated view of the role of cholesterol in multiple diseases. Yoon, H. Lipid metabolism in sickness and in health: emerging regulators of lipotoxicity. Cell 81 , — Garbarino, J. Sterol and diacylglycerol acyltransferase deficiency triggers fatty acid-mediated cell death.
Chitraju, C. Triglyceride synthesis by DGAT1 protects adipocytes from lipid-induced ER stress during lipolysis. Kuo, A. Lipid droplet biogenesis and function in the endothelium. Liu, L. DGAT1 expression increases heart triglyceride content but ameliorates lipotoxicity.
Long, M. DGAT1 activity synchronises with mitophagy to protect cells from metabolic rewiring by iron depletion. Kwiatek, J. Acta , Rambold, A. Fatty acid trafficking in starved cells: regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics.
Nguyen, T. DGAT1-dependent lipid droplet biogenesis protects mitochondrial function during starvation-induced autophagy. Cell 42 , 9— e5 Hosios, A. mTORC1 regulates a lysosome-dependent adaptive shift in intracellular lipid species.
Han, J. The role of ER stress in lipid metabolism and lipotoxicity. Lin, L. cPLA2 is phosphorylated and activated by MAP kinase. Cell 72 , — Gubern, A. Group IVA phospholipase A2 is necessary for the biogenesis of lipid droplets.
Darling, N. The role of MAPK signalling pathways in the response to endoplasmic reticulum stress. Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell , — Bailey, A. Antioxidant role for lipid droplets in a stem cell niche of Drosophila.
Roberts, M. Protein quality control and lipid droplet metabolism. Palmitate-induced apoptosis can occur through a ceramide-independent pathway.
Park, C. Dysregulation of lipid droplet protein expression in adipose tissues and association with metabolic risk factors in adult females with obesity and type 2 diabetes.
Dahlman, I. The CIDEA gene VF polymorphism is associated with obesity in Swedish subjects. Diabetes 54 , — Wu, J.
The genetic contribution of CIDEA polymorphisms, haplotypes and loci interaction to obesity in a Han Chinese population. Zhou, Z. Cidea -deficient mice have lean phenotype and are resistant to obesity.
Li, J. Cideb regulates diet-induced obesity, liver steatosis, and insulin sensitivity by controlling lipogenesis and fatty acid oxidation. Diabetes 56 , — Nishino, N. FSP27 contributes to efficient energy storage in murine white adipocytes by promoting the formation of unilocular lipid droplets.
CAS PubMed PubMed Central Google Scholar. Martinez-Botas, J. Tansey, J. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity.
USA 98 , — Sun, Z. Perilipin1 promotes unilocular lipid droplet formation through the activation of Fsp27 in adipocytes. Puri, V. Lipid droplets: FSP27 knockout enhances their sizzle.
Qian, K. CLSTN3β enforces adipocyte multilocularity to facilitate lipid utilization. James, D. The aetiology and molecular landscape of insulin resistance.
Virtue, S. PLoS Biol. Daemen, S. Blüher, M. Metabolically healthy obesity. Stefan, N. Metabolically healthy and unhealthy normal weight and obesity.
Korea 35 , — Garg, A. Acquired and inherited lipodystrophies. Gonzaga-Jauregui, C. Clinical and molecular prevalence of lipodystrophy in an unascertained large clinical care cohort. Diabetes 69 , — Lim, K. Zammouri, J. Molecular and cellular bases of lipodystrophy syndromes.
Fourman, L. Approach to the patient with lipodystrophy. Patni, N. Lipodystrophy for the diabetologist — what to look for. Simha, V. Phenotypic heterogeneity in body fat distribution in patients with congenital generalized lipodystrophy caused by mutations in the AGPAT2 or seipin genes.
Gale, S. A regulatory role for 1-acylglycerolphosphate-O-acyltransferase 2 in adipocyte differentiation. Subauste, A. Alterations in lipid signaling underlie lipodystrophy secondary to AGPAT2 mutations.
Diabetes 61 , — Congenital generalized lipodystrophies — new insights into metabolic dysfunction. Cui, X. Seipin ablation in mice results in severe generalized lipodystrophy. Diabetes 63 , — Mak, H. AGPAT2 interaction with CDP-diacylglycerol synthases promotes the flux of fatty acids through the CDP-diacylglycerol pathway.
Blouin, C. Lipid droplet analysis in caveolin-deficient adipocytes: alterations in surface phospholipid composition and maturation defects. Riazi, K. Race and ethnicity in non-alcoholic fatty liver disease NAFLD : a narrative review.
Nutrients 14 , Yki-Järvinen, H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. Targher, G. Non-alcoholic fatty liver disease: a multisystem disease requiring a multidisciplinary and holistic approach.
Lancet Gastroenterol. Eslam, M. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology , — e1 Sheka, A. Nonalcoholic steatohepatitis: a review. JAMA , — Day, C. Gastroenterology , — Buzzetti, E.
The multiple-hit pathogenesis of non-alcoholic fatty liver disease NAFLD. Metabolism 65 , — Tilg, H. Multiple parallel hits hypothesis in nonalcoholic fatty liver disease: revisited after a decade. Hepatology 73 , — Barrows, B. Contributions of different fatty acid sources to very low-density lipoprotein-triacylglycerol in the fasted and fed states.
Donnelly, K. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. Das, K. Lean NASH: distinctiveness and clinical implication. Mashek, D. Hepatic lipid droplets: a balancing act between energy storage and metabolic dysfunction in NAFLD.
Smith, G. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. Hodson, L. The regulation of hepatic fatty acid synthesis and partitioning: the effect of nutritional state. Scorletti, E. A new perspective on NAFLD: focusing on lipid droplets.
Organellar proteomics and phospho-proteomics reveal subcellular reorganization in diet-induced hepatic steatosis.
Romeo, S. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Yuan, X. Population-based genome-wide association studies reveal six loci influencing plasma levels of liver enzymes.
Chen, W. Hepatology 52 , — Basantani, M. He, S. A sequence variation IM in PNPLA3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis. Huang, Y. Expression and characterization of a PNPLA3 protein isoform IM associated with nonalcoholic fatty liver disease.
BasuRay, S. The PNPLA3 variant associated with fatty liver disease IM accumulates on lipid droplets by evading ubiquitylation.
Hepatology 66 , — Accumulation of PNPLA3 on lipid droplets is the basis of associated hepatic steatosis. Wang, Y. PNPLA3, CGI, and inhibition of hepatic triglyceride hydrolysis in mice. Hepatology 69 , — Yang, A.
Dynamic interactions of ABHD5 with PNPLA3 regulate triacylglycerol metabolism in brown adipocytes. Schott, M. Lipid droplet size directs lipolysis and lipophagy catabolism in hepatocytes. Kabbani, M. Human hepatocyte PNPLAM exacerbates rapid non-alcoholic fatty liver disease development in chimeric mice.
Pirazzi, C. PNPLA3 has retinyl-palmitate lipase activity in human hepatic stellate cells. Bruschi, F. PNPLA3 expression and its impact on the liver: current perspectives.
Hepatic Med. Ma, Y. Abul-Husn, N. A protein-truncating HSD17B13 variant and protection from chronic liver disease. Seko, Y. Attenuated effect of PNPLA3 on hepatic fibrosis by HSD17B13 in Japanese patients with non-alcoholic fatty liver disease.
Liver Int. Su, W. Comparative proteomic study reveals 17β-HSD13 as a pathogenic protein in nonalcoholic fatty liver disease. Phosphorylation of 17β-hydroxysteroid dehydrogenase 13 at serine 33 attenuates nonalcoholic fatty liver disease in mice.
Ng, S. Convergent somatic mutations in metabolism genes in chronic liver disease. Verweij, N. Germline mutations in CIDEB and protection against liver disease. Xu, W. Differential roles of cell death-inducing DNA fragmentation factor-α-like effector CIDE proteins in promoting lipid droplet fusion and growth in subpopulations of hepatocytes.
Ye, J. Cideb, an ER- and lipid droplet-associated protein, mediates VLDL lipidation and maturation by interacting with apolipoprotein B. CideB protein is required for the biogenesis of very low density lipoprotein VLDL transport vesicle.
Su, L. Kozlitina, J. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Mancina, R. The MBOAT7-TMC4 variant rs increases risk of nonalcoholic fatty liver disease in individuals of European descent.
Baselli, G. Rare ATG7 genetic variants predispose patients to severe fatty liver disease. de Vree, J. Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis. Tanoli, T. Fatty liver in familial hypobetalipoproteinemia: roles of the APOB defects, intra-abdominal adipose tissue, and insulin sensitivity.
Ward, L. GWAS of serum ALT and AST reveals an association of SLC30A10 Thr95Ile with hypermanganesemia symptoms. Faulkner, C. A single nucleotide polymorphism of PLIN2 is associated with nonalcoholic steatohepatitis and causes phenotypic changes in hepatocyte lipid droplets: a pilot study.
Najt, C. Perilipins at a glance. Wagner, R. Metabolic implications of pancreatic fat accumulation. Schaefer, J. The normal weight of the pancreas in the adult human being: a biometric study.
Wong, V. Fatty pancreas, insulin resistance, and β-cell function: a population study using fat-water magnetic resonance imaging.
Singh, R. Ectopic fat accumulation in the pancreas and its biomarkers: a systematic review and meta-analysis. Diabetes Metab. Prentki, M. Lipid-associated metabolic signalling networks in pancreatic beta cell function.
Diabetologia 63 , 10—20 Smits, M. The clinical significance of pancreatic steatosis. Ogilvie, R. The islands of Langerhans in 19 cases of obesity. Olsen, T. To understand how lipid dysmetabolism may contribute to ALS pathogenesis, we have summarized the pertinent ALS metabolomic and lipidomic findings of broad lipid classes from human patient samples and animal models in Tables 1 , 2 , 3.
A lipid class is marked to have higher or lower levels if the majority of significantly differentially expressed species in the lipid class are upregulated or downregulated in ALS patients as compared to healthy controls; otherwise, it is marked as mixed expression.
This section discusses the ALS lipidomic signatures by tissue type, and potential lipid biomarkers identified. We will discuss each lipid class in more detail in the later sections. Given the non-invasive nature, blood sampling from patients is an ideal source for biomarkers.
Not surprisingly, many of the ALS metabolite studies have investigated blood samples from patients Table 1. Due to the blood—brain barrier BBB and blood-spinal cord barrier BSCB , which limit the exchange of metabolites between the CNS and the peripheral blood, there are two obvious caveats to be considered.
The first is whether the changes in blood lipids are a reflection of or a response to the changes in the CNS or vice versa? The second is how leaky BBB and BSCB during ALS progression [ 58 , 59 ] contribute to the changes in blood and brain lipids?
The latter point would also inevitably introduce greater variations among different studies. An early metabolomics study of plasma samples from ALS patients identified metabolites. The panel of 32 metabolites contained 11 highly expressed lipids, including saturated fatty acids, arachidonic acid, SM, cholesterol, and cortisone.
The levels of the panel lipids correlated with disease severity tested by the ALS functional rating scale ALSFRS-R score [ 34 ]. Subsequent studies using plasma samples with a wider detection range show similar trends of higher expression levels of a large number of SM and fatty acid species [ 60 , 61 , 62 ].
Goutman et al. additionally identified higher levels of ceramides, glucosylceramides, lactosylceramides, diacylglycerides and lysophospholipids [ 60 ]. A two-year longitudinal lipidomic study investigated serum samples from patients with ALS or primary lateral sclerosis PLS , a motor neuron disease that targets upper motor neurons.
The two diseases have similar lipid profiles, but could be distinguished by dysregulation of glycerophospholipids in ALS that is not seen in PLS [ 62 ].
ALS patient serum also has elevated expression of specific cholesterol esters, ceramides and SM species that correlates with disease progression, which was not observed in the PLS patients [ 62 ]. However, another lipidomic study using fasting serum could not accurately discriminate ALS patients from healthy controls despite changes in lipid profiles [ 63 ].
Nevertheless, it identified four lipids that have consistently higher expression in ALS patient serum, including two monounsaturated fatty acids and , a TG TG and a SM SM [ 63 ]. Plasma samples from pre-symptomatic individuals, who developed ALS within five years of sample collection, showed mild dysregulation of glycerolipids, cholesterol esters, PC, and SM.
However, no significant changes or signatures could be identified to predict diseased individuals [ 64 ]. The other biofluid of interest in ALS studies is the CSF, which may contain lipids released by damaged cells under the diseased state.
The ALS CSF lipidomic signature is distinct from the plasma, with only 19 and 17 differential lipids identified in two studies [ 61 , 65 ] Table 2.
The most discriminant molecule in the CSF is the increased PC [ 65 ], which is also observed in the brains of SOD1-G93A ALS mice [ 66 ]. SM and glucosylceramides are also observed to be elevated in the CSF [ 65 ]. Degeneration of spinal cord motor neurons is a characteristic of ALS.
Therefore, spinal cord is expected to be the site of high metabolite dysregulation. Lipidomic studies from spinal cords of ALS patients and ALS mouse models are summarized in Tables 2 and 3. Targeted mass spectrometry studies of spinal cord tissues from postmortem ALS patients showed elevated levels of cholesterol esters and a range of sphingolipids including SM, ceramides, glucosylceramide, galactosylceramide, lactosylceramide, globosides and gangliosides [ 33 , 36 ].
In addition, the spinal cord gray matter has elevated levels of TGs and lysoPC [ 35 ]. Some of these changes have also been observed in ALS mouse models. The spinal cords of SOD1-G93A mouse model show elevated levels of specific ceramides, glucosylceramide, gangliosides and cholesterol esters [ 33 , 36 ], whereas the SOD1-G86R mice have elevated levels of gangliosides and phosphatidylinositol and lower levels of ceramides and glucosylceramides [ 67 ].
The ALS FUS mouse model which overexpresses wild-type human FUS has elevated levels of cholesterol esters and specific ceramides, and dysregulation of phospholipids, including lower levels of cardiolipin [ 68 ].
Downregulation of cardiolipins has also been observed in SOD1-G86R rat spinal cords, along with a nearly six-fold increase in cholesterol esters [ 69 ]. Lipid composition changes in the isolated nuclei of the spinal cord from ALS patients include altered expression of diglycerides, TGs, plasmalogens and glycerophospholipids, which are known to be major components of the nuclear membrane [ 70 ].
This suggests changes in lipid composition of the nuclear membrane and nucleoplasm in the ALS neurons. ALS patient fibroblasts and iPSC-derived neurons have also been studied in terms of lipid profiling.
ALS patient fibroblasts display many mitochondrial defects similar to those found in ALS motor neurons, and have been used to study mitochondrial metabolism and to discover biomarkers for ALS [ 71 , 72 ].
The lipidome of skin fibroblasts from ALS patients has elevated levels of SM, ceramides, and phospholipids [ 73 ].
Among them, PC is also observed to be highly discriminatory in CSF of ALS patients [ 61 ] and mouse models [ 66 ]. A recent study analyzed ALS patient iPSC-derived neurons using multi-omics approaches, including genomics, proteomics, and metabolomics.
Higher levels of arachidonic acids and phospholipids such as PE, PS, phosphatidylglycerol, and lysophospholipids, are observed in spinal cord motor neuron cultures compared to ocular motor neuron cultures derived from human ALS-iPSCs cell lines [ 18 ].
Among the lipid species, the elevated arachidonic acid has been proposed to play a role in the selective vulnerability of spinal cord motor neurons in ALS [ 18 ]. Besides spinal cords, lipidomic studies have also been carried out in muscles and motor cortex tissues from SOD1-G86R mice [ 67 ] and SOD1-G86R rats [ 69 ], respectively.
Lipidomic analysis of skeletal muscles from SOD1-G86R mice revealed similar findings as the spinal cord signatures of ALS patients, with increased levels of ceramides and glucosylceramide and dysregulation of phospholipids [ 67 ].
It should be noted that progressive loss of neuromuscular junctions NMJs is a key pathological feature in ALS [ 74 ]. While whether ALS genetic risk factors would intrinsically affect muscle function and degeneration remains to be addressed, muscles and NMJs remain a primary and attractive site for therapeutic intervention [ 75 , 76 ].
Concerning the motor cortex, SOD1-G86R rats show increased levels of ceramides, glucosylceramide and phospholipids in the motor cortex [ 69 ]. However, changes in the lipid composition are mostly associated with age rather than with the disease symptomatic stage [ 69 ].
Overall, these lipidomic changes remain largely distinct between various disease samples. This could be due to the differences in disease status, CNS and peripheral systems, affected cell and tissue types, etc. Furthermore, there could be some compensatory mechanisms, such as the regulation of lipid transport, uptake, and utilization.
Nevertheless, common themes may be emerging from these studies. In the following, we will discuss lipidomic changes observed in each lipid class and sub-class in detail.
In the CNS, excess fatty acids are stored as lipid droplets primarily in astrocytes, and the lipid droplet formation is increased in response to hypoxia, cellular stress, and exposure to high levels of exogenous free fatty acids.
Fatty acid oxidation produces more energy as compared to glucose, but also takes up more oxygen resources. Thus, prolonged usage of fatty acid β-oxidation places cells under oxidative stress, leading to the production of harmful reactive-oxygen species.
Unlike neurons, astrocytes generate a large number of antioxidant molecules and are also the major site of lipid storage and oxidation in the CNS [ 78 ]. It has been proposed that due to the high energy demand and impaired glucose metabolism in ALS, there is a switch to using lipids as an energy source via fatty acid oxidation in both neurons and astrocytes.
This switch in energy source may place the system under elevated oxidative stress, thereby contributing to motor neuron death in ALS [ 23 , 79 , 80 ].
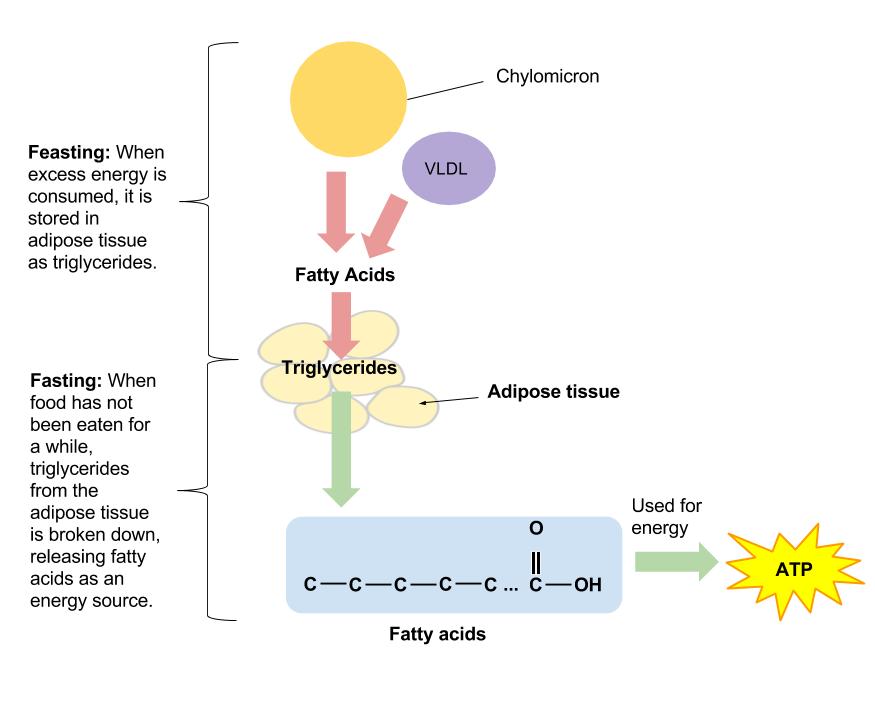
Fatty acids are stored as TGs in lipid droplets, and released for utilization under starvation conditions [ 55 , 81 ].
A recent study showed that C9ORF72, whose hexanucleotide repeat expansion is causal for ALS [ 82 , 83 ], regulates lipid metabolism under starvation conditions [ 84 ]. Specifically, C9ORF72 deletion leads to reduced lipid droplets and increased de novo fatty acid synthesis under starvation conditions, accompanied by upregulation of NOX2.
NOX2 is a NADH oxidase that is known to cause oxidative stress and has been shown to be upregulated in ALS patients [ 85 , 86 ]. This C9ORFdependent starvation-related lipid dysmetabolism is mediated by preventing the degradation of coactivator-associated arginine methyltransferase 1 CARM1.
Furthermore, CARM1 upregulation and C9ORF72 reduction are observed in the spinal cords of C9ORFlinked ALS patients [ 85 , 86 ]. Taken together, these findings further support the notion that increased fatty acid utilization as an energy source in ALS may lead to excess oxidative stress.
However, fatty acids represent more than an energetic support for neural cells. Emerging evidence suggests that fatty acids may play important roles in ALS disease progression.
As discussed above, greater fatty acid metabolism triggers increased oxidative stress, leading to production of peroxidated toxic lipids. These peroxidated lipids are released from neurons and taken up by astrocytes, which either break down the lipids or store them as lipid droplets.
This neuron-astrocyte coupling of lipid metabolism is protective for neurons [ 78 , 87 ]. Interestingly, Guttenplan et al. demonstrated that neurotoxic reactive astrocytes secrete long-chain saturated free fatty acids that contribute to cell death [ 88 ].
Fractionation of conditional media of neurotoxic reactive astrocytes with column chromatography led to identification of apolipoprotein E APOE - and apolipoprotein J-containing lipoprotein particles with long-chain saturated free fatty acids, which contribute to the observed toxicity.
Consistently, unbiased lipidomics of more than lipids from 10 classes revealed significant upregulation of long-chain saturated free fatty acids in the conditioned media of these reactive astrocytes.
The conditional media from ELOVL1-KO astrocytes were less toxic than that of wild-type mice, indicating that the long-chain saturated free fatty acids secreted by astrocytes trigger cell death [ 88 ].
In the context of ALS, although higher levels of saturated fatty acids have been reported in the plasma of ALS patients [ 34 , 60 ], it is not known whether there is enhanced expression or activity of astrocytic ELOVL1.
Furthermore, whether ALS astrocytes also secrete more long-chain free fatty acids that may be part of astrocyte-induced toxicity in ALS remains to be addressed. Thus, most unsaturated fatty acids are liquids at room temperature, which has many biological implications such as maintaining membrane order and fluidity, and the positions of double bonds affect function [ 89 ].
Unsaturated fatty acids are sub-grouped by the number of double bonds into monounsaturated fatty acids MUFA and polyunsaturated fatty acids PUFA. The conversion of MUFAs to saturated fatty acids, catalyzed by stearoyl-CoA desaturase SCD , is sensitive to energy metabolism requirements of the body.
By contrast, other markers that are used in the measurement for obesity, such as body mass index or leptin concentration, do not correlate.
Interestingly, a MUFA-enriched diet ameliorates disease symptoms and increases the survival rate of ALS SOD1-G93A mouse model [ 92 ]. However, higher plasma and serum levels of MUFA have been reported in ALS patients [ 34 , 60 , 61 , 62 ], and the MUFAs C and C are consistently increased in ALS patient serum samples [ 63 ].
However, the exact role of MUFAs in ALS remains to be clarified. PUFAs have been proposed to mediate motor neuron toxicity in ALS and regulate inflammatory responses, with elevated levels observed in ALS patient plasma [ 34 , 60 , 61 , 62 ]. Omega-3 ω-3 and omega-6 ω-6 fatty acids, i.
ω-3 fatty acids, such as eicosapentaenoic acid EPA, ω-3 and docosahexaenoic acid DHA, ω-3 , give rise to anti-inflammatory eicosanoids.
By contrast, ω-6 PUFAs, such as linoleic acid ω-6 and arachidonic acid ω-6 , give rise to pro-inflammatory eicosanoids [ 93 ]. Since mammals are unable to convert ω-6 PUFAs to ω-3 PUFAs, or synthesize PUFAs de novo, tissue levels of these PUFAs and their associated eicosanoids are directly linked to their dietary intake [ 43 , 94 ].
Dietary supplementation of ω-3 fatty acids has been shown to offer various benefits in rodents, including reduced neuroinflammation and improved spatial memory [ 95 ].
Consistent with the potential beneficial role of ω-3 fatty acids, a longitudinal study using questionnaire data on ALS patients concluded that a lower risk of ALS is associated with a greater intake of ω-3 fatty acids [ 96 ]. However, dietary supplementation of EPA accelerates disease progression and shortens the lifespan of SOD1-G93A mice, despite decreased neuroinflammation [ 97 ].
Intriguingly, another study in SOD1-G93A mice demonstrated that higher supplementation of ω-6 fatty acids delays disease progression, while dietary supplementation with equal amounts of ω-3 and ω-6 fatty acids accelerates disease progression and death [ 98 ]. These studies suggest that a fine balance of ω-3 and ω-6 may need to be maintained, and disruptions of the intake ratio between ω-3 and ω-6 may influence ALS disease progression.
Arachidonic acid an ω-6 PUFA and DHA an ω-3 PUFA are two major PUFAs present in high concentrations in the CNS. DHA has been reported to be elevated in the brains and spinal cords of ALS patients [ 99 ].
Arachidonic acid, which is found at higher levels in plasma of ALS patients, is part of a metabolite panel that is not only discriminatory but also positively correlates with the disease severity [ 34 ].
One mechanism to increase arachidonic acid could be the hydrolysis of membrane phospholipids by cytosolic phospholipase A2 cPLA2.
In this regard, the expression and activity of cPLA2 are elevated in the spinal cords of ALS patients [ ], as well as in motor neurons of SOD1-G93A mice [ ].
Furthermore, the released arachidonic acid may then be metabolized by cyclooxygenases COXs , lipoxygenases LOXs and cytochrome P enzymes into multiple biologically active eicosanoids, such as prostaglandins, thromboxanes, prostacyclins, and leukotrienes, which also act as mediators of inflammatory response.
Not surprisingly, elevated levels of arachidonic acid have been proposed to contribute to neurotoxicity via elevated neuroinflammation. They are potent, but short-lived, cell signaling molecules involved in regulating inflammation, immune response, pain perception, and allergies [ 43 , 94 ]. They are most frequently derived from arachidonic acid, followed by EPA, and are the mediators of the effects of PUFAs.
Arachidonic acid can be metabolized by 5-liopxygenase to generate leukotrienes LTs , such as 5-hydroperoxy-6,8,11,14 eicosatetraenoic acid 5-HPETE and subsequently other LTs, or by cyclooxygenases 1 and 2 COX1, consecutively expressed in most of tissues, and COX2, inducible expression to generate prostaglandins and thromboxanes [ ] Fig.
Arachidonic acid-derived eicosanoids have been studied extensively in ALS and are discussed below. A recent study by Lee and colleagues provided further evidence that the arachidonic acid pathway contributes to motor neuron dysfunction and death in ALS [ 18 ].
Using patient-derived iPSC harboring ALS mutations, Lee and colleagues compared the molecular signatures of derived spinal motor neurons affected cell type in ALS and ocular motor neurons unaffected cell type in ALS. Co-analysis of transcriptomics and metabolomics data identified activation of the arachidonic acid pathway as a common feature of ALS spinal motor neurons.
In particular, metabolomic analyses revealed significant down-regulation of a 5-lipoxygenase 5-LOX inhibitor analog in ALS spinal motor neuron cultures. Indeed, when testing known 5-LOX inhibitors as potential therapeutic agents in ALS iPSC-derived motor neurons, a Drosophila model of C9ORF72 ALS and SOD1-G93A mice, the results showed promotion of survival [ 18 ].
Profiling of arachidonic acid derivatives show increased levels of LOX-derived metabolites, such as hydroxy-eicosatetraenoic acids hydroxyeicosatetraenoic acid and hydroxyeicosatetraenoic acid , and COX-derived prostaglandins prostaglandin E2 [PGE 2 ] and Prostaglandin D2 and thromboxane B2, in SOD1-G93A mice [ ].
Increased levels of PGE 2 and enzymes involved in its biosynthesis and metabolism have been reported in serum and CSF of ALS patients as well as in spinal cords of SOD1-G93A mice [ , , , ].
A pilot study in 50 sporadic ALS patients and controls found elevated F2t-isoprostane in urine samples of the ALS patients [ ]. A positive correlation has been found between increased urinary concentration of a prostaglandin D2 metabolite, 11,dioxohydroxy-,2,3,4,5-tetranorprostan-1,dioic acid, and ALS progression [ ].
However, the study was limited to only six ALS patients and controls [ ]. Recently, endocannabinoids, also derivatives of PUFAs, have received attention for ALS therapeutics. They are naturally occurring eicosanoid sub-family molecules and act as non-classical retrograde neurotransmitters, which bind and activate the cannabinoid receptors 1 and 2 CB1 and CB2, respectively [ ].
Activation of cannabinoid receptors in turn activates the anti-glutamatergic and anti-inflammatory responses, which are neuroprotective in nature [ 44 , , ].
Arachidonoyl ethanolamide AEA also called anandamide and 2-arachidonoyl glycerol 2-AG are two endocannabinoids abundant in humans [ 44 , ] Fig.
Furthermore, serum concentrations of AEA and 2-AG were found to be elevated and predict ALS in a study of 47 ALS patients and controls [ ]. Upregulation of CB2 receptor has been reported in ALS patient spinal cords and motor cortex [ ], and in spinal cord of a canine ALS model [ ]. Glycerolipids are neutral lipids and act as precursors to other lipids in the CNS.
They are most abundantly found in astrocytes within lipid droplets [ 55 , 81 ]. Glycerolipids can be phosphorylated to form glycerophospholipids, or hydrolyzed to give rise to fatty acids of various chain lengths. In high energy demand conditions, glycerolipids are quickly depleted to produce fatty acids for energy metabolism.
ALS patients with elevated serum TG levels are reported to have prolonged life expectancy, suggesting that serum level of TGs could be used as a prognostic factor [ 22 ]. Lipidomic studies have reported higher levels of diglycerides [ 60 , 62 ] and TGs [ 61 , 62 , 63 ] in the sera of ALS patients, of which the TG is a reliable discriminant lipid to distinguish patient samples from healthy controls.
A longitudinal study with a two-year follow-up after the first measurement, found increased diglyceride and TG levels but decreased monoglyceride levels in ALS patients at later stages of the disease [ 62 ].
Interestingly, the diglyceride species containing MUFAs like palmitoleic and oleic acids are significantly elevated. TGs containing the same MUFAs are also elevated though not significantly.
Elevated levels of diglycerides, which are precursors to TGs in serum, suggest increased de novo glyceride synthesis, or mobilization from adipose tissue, or both. Additionally, in the plasma, TG levels are found to be associated with serum levels of neurofilament, an established neuronal damage marker [ 61 ].
Furthermore, TG levels of C16 and C species are increased up to three folds in spinal cords of male ALS patients and are also elevated in spinal cords of SOD1-G93A mice [ 35 ].
In mouse spinal cords, TG accumulation increases with disease progression and is predominant in gray matter [ 35 ].
However, it remains to be determined if these observations indicate a greater consumption of glycerolipids in the CNS in order to meet the energy demand and if there is a switch to fatty acid oxidation.
Glycerophospholipids are major structural components of all eukaryotic plasma membranes [ 50 , 51 ]. Phospholipids form the characteristic phospholipid bilayer, and their composition affects membrane geometry, fluidity, and permeability [ 50 , 51 ].
Some species also function as bioactive signaling molecules. PC is a main source of acetylcholine in the CNS, and intake of PC can improve memory and learning and ameliorate cognitive decline in mouse models of dementia [ , , ].
Elevated levels of PC have been reported in the CSF [ 65 ] and the spinal cord nuclear lipidome [ 70 ] of ALS patients, the spinal cords of FUS overexpression mice [ 68 ], the skeletal muscle of SOD1-G86R mice [ 67 ], and the motor cortex of SOD1-G93A mice [ 69 ]. Elevated levels of PC and PC have been found to be the most discriminatory in the CSF and plasma, being able to differentiate between slow- and fast-progression cases [ 61 , 65 ].
Elevated levels of PC are also observed in SOD1-G93A mouse brains [ 66 ]. In a longitudinal study of ALS patients across two years, several species of PC and PS were decreased in patient blood in the initial stage of pathogenesis, with reductions in PS and PS being discriminatory even at baseline.
Follow-up samples had elevated levels of these PCs, suggesting an increased level with disease progression. Despite an initial decline, PE levels increased progressively in the ALS patients and PE could even be used to discriminate ALS from PLS [ 62 ].
Lysophospholipid levels are reported to be elevated in plasma [ 34 , 60 , 61 , 63 ], CSF [ 65 ] and spinal cords [ 35 , 70 ] of ALS patients, in the spinal cords of FUS-overexpression mice [ 68 ], and in skeletal muscles of SODR mice [ 67 ].
Lyso-PC is commonly discriminatory in ALS patient CSF and SOD1-G93A mice brains, along with elevation of lysoPCs containing the long-chain fatty acids C, C and C [ 65 ]. Specific species of lysoPC esters and lysoPE plasmalogens are significantly and progressively reduced in ALS patient blood samples [ 62 ].
A study testing for levels of lysoPCs containing fatty acids with various saturation status reported an increase of lysoPCs containing C16 and Cn9 fatty acids in spinal cords of both ALS patients and SOD1-G93A mice [ 35 ]. Lyso-PCs are generally a by-product of cholesterol ester synthesis that is elevated in ALS conditions.
Addition of these lysoPC species C16, C18, and Cn9 to motor neuron cultures in vitro causes motor neuron toxicity and death compared to their corresponding free fatty acids C16, C18, C as controls, with lysoPC C16 being more toxic than others [ 35 ].
The data suggest that the elevated lysoPC level may be detrimental for motor neurons. Sphingolipids are a class of lipids which are ubiquitously found in cell membranes and are an integral constituent of lipid rafts, contributing to membrane stability and permeability [ 50 , ].
Sphingolipids are highly enriched in the CNS, where different CNS cell types have different sphingolipid profiles [ ]. Due to this diverse distribution, sphingolipids have been shown to be vital in brain development, neurogenesis, differentiation, axonal growth and ageing [ 46 , , ].
Breakdown of sphingolipids takes place in the lysosome and defects in this process can lead to accumulation of sphingolipids, which is implicated in many neurological diseases, including ALS. Mutations in enzymes catalyzing the degradation of these sphingolipids are responsible for a large group of lysosomal storage diseases, also called sphingolipidosis [ ], which is often manifested as ALS-like symptoms see below.
Collectively, these observations suggest that homeostatic regulation of sphingolipid metabolism is essential for CNS function Fig. Ceramides consist of a sphingosine attached to a fatty acid tail, and are the precursors to the more complex sphingolipids Fig.
They are primarily generated by de novo synthesis, and from the breakdown of more complex sphingolipids, especially the breakdown of SM.
The first and rate-limiting step of de novo ceramide synthesis is the condensation of palmitoyl-CoA and L -serine, catalyzed by SPT, to form 3-keto-sphinganine, which is reduced to sphinganine, a key intermediary. Sphinganine is N -acylated by one of the ceramide synthases CerS , each of which has a preferential specificity for fatty acyl CoAs of different carbon-chain lengths, leading to the formation of dihydroceramides with different chain lengths, which are then desaturated to form ceramides [ 45 , , ].
An early milestone study on the role of sphingolipids in ALS was published in [ 33 ]. Cutler and colleagues quantified various lipids in ALS patient spinal cords, and found accumulations of ceramides, SM, and cholesterol esters along with increased oxidative stress.
Similar results were obtained at the pre-symptomatic stage in the spinal cords of SOD1-G93A mice. To understand the relationship among oxidative stress, deficits in sphingolipid biosynthesis and cell death, cultured motor neurons were treated with either DMNQ, an oxidative stress-inducing agent, or palmitoyl-CoA, the initial substrate for sphingolipid synthesis catalyzed by SPT.
Either DMNQ or palmitoyl-CoA treatment alone increased the levels of ceramide and cholesterol esters within 6 h of exposure and triggered a dose-dependent cell-death. Combination of DMNQ and palmitoyl-coA resulted in exacerbated increase of ceramides, SM, cholesterol esters and cell death, all of which were reduced on treatment with DMNQ and an SPT inhibitor [ 33 ].
Thus, their data suggest that oxidative stress acts through enhanced sphingolipid synthesis to induce neuronal death. Furthermore, ceramide induces apoptotic cell death in cortical and motor neurons [ , , , ], suggesting that accumulation of ceramides could contribute to ALS pathogenesis [ 33 ].
Indeed, elevated levels of ceramide have been reported in spinal cords and motor cortex of SOD1-G93A rats [ 69 ], as well as in plasma [ 60 , 62 , 63 ], spinal cords [ 33 , 36 ] and fibroblasts [ 73 ] of ALS patients.
Furthermore, increased ceramide levels are observed in spinal motor neurons, but not in ocular motor neurons, derived from ALS patients [ 18 ].
Taken together, these data suggest that accumulation of ceramide, a precursor for sphingolipids, could contribute to ALS pathogenesis. Recent whole-exome sequencing in juvenile- and adult-onset ALS patients identified several genetic variants of SPTLC1 [ 16 , 17 ].
SPTLC1 is the long-chain subunit 1 of the enzyme SPT, which is the rate-limiting enzyme for ceramide biosynthesis. At least two not-mutually-exclusive mechanisms have been proposed for these dominant-acting SPTLC1 variants.
The first mechanism takes cue from hereditary sensory neuropathy type1 HSAN1 , a disease characterized by atrophy of sensory neurons [ , ]. Mutations in SPTLC1 are the underlying cause of HSAN1. In HSAN1, the SPTLC1 variants cause alterations of substrate specificity of the SPT enzyme from L -serine to either L -alanine or L -glycine, leading to the formation of 1-deoxysphingolipids instead of ceramides.
These atypical 1-deoxysphingolipids cannot be synthesized into more complex sphingolipids or degraded, thereby resulting in accumulation of atypical 1-deoxysphingolipids that are highly neurotoxic [ ]. HSAN1 patients are often treated with oral supplementation of L -serine to reduce production of the toxic 1-deoxysphingolipids [ ].
The p. SY SPTLC1 variant [ 16 ] identified in juvenile ALS has been previously reported as an atypical HSAN1 variant with a distinct mixed sensorimotor neuropathy phenotype [ ].
Furthermore, using cell culture assays, Johnson et al. reported that the p. A20S SPTLC1 variant also showed an altered substrate preference for L -alanine and L -glycine, along with mitochondrial defects, which were rescued on exposure to L -serine [ 16 ].
Thus, these observations indicate that the ALS-linked variants alter substrate specificity of SPT [ , ]. The other juvenile ALS variants identified are distinct from HSAN1 variants and map to exon 2 of SPTLC1, including p. A20S, p. Y23F, p. L39del and p.
This exon codes for a transmembrane domain that interacts with ORMDL proteins to inhibit SPT activity [ 17 , ]. Mohassel et al. showed that these juvenile ALS variants are not sensitive to ORMDL protein levels, resulting in higher levels of sphinganine and ceramides.
Correspondingly, increased levels of ceramides, but not 1-deoxysphingolipid a HSAN1 characteristic feature , are found in the sera of juvenile ALS patients with variants p. It should be noted that Mohassel et al. did not test substrate specificity preferences of the variants.
Furthermore, selective knockdown of the ALS SPTLC1 allele restored normal ceramide levels in human iPSC motor neurons [ 17 ]. Thus, these ALS-linked variants of SPTLC1 could disrupt regulation of SPT, resulting in unrestrained activity and higher ceramide levels.
All the evidence presented above indicates that increased accumulation of ceramides is a common theme for ALS. Thus, prevention of ceramide accumulation could be a potential therapeutic intervention and can be achieved by promoting its synthesis to other sphingolipids or its degradation.
Indeed, inhibition of glucosylceramide synthase GCS , the enzyme which synthesizes glucosylceramide from ceramides, accelerates disease progression in SOD1-G93A mice [ 36 ]. Genetic deficiency of ceramidase, the enzyme which degrades ceramides, is causal to Farber disease, a lipid storage disease with some patients exhibiting muscle weakness and seizures [ ].
As the name suggests, SMs are abundantly present in the myelin sheath. They are the most abundant sphingolipid found in cell membranes and play a critical role in maintaining myelin sheath integrity and function and in neuroinflammation and signal transduction [ 51 , , , , ]. Sphingomyelinase encoded by SMPD1 breaks down SM into ceramide and phosphocholine.
Mutations in SMPD1 cause accumulation of SM in the CNS, leading to dementia, ataxia, and slurred speech as seen in Niemann-Pick disease type A and B [ , , ] Fig. SM accumulation has been reported in plasma [ 34 , 60 , 61 , 63 ], CSF [ 65 ], spinal cords [ 33 , 36 ] and fibroblasts [ 73 ] of ALS patients.
Elevated levels of SM , SM , SM OH, and SM in the plasma are found to be accurate discriminators of ALS disease progression and predictors of clinical indicators in a study with 74 ALS patients [ ]. Area-Gomez et al. additionally reported significantly lower levels of SM , SM and SM species in serum samples from ALS patients [ 62 ].
The expression profiling of SMs in ALS mouse models remains inconclusive. Cutler et al. reported an increase of SM in the spinal cords of SOD1-G93A mice from pre-symptomatic to post-symptomatic stage [ 33 ].
By contrast, Dodge et al. reported an initial decrease of SM at paralysis onset, but a normal sphingomyelin level at full-paralysis stage in the spinal cords of SOD1-G93A mice [ 36 ].
The expression levels of SM are mixed in SOD1-G86R mouse spinal cord and skeletal muscle [ 67 ], and are reported to be lower in mice overexpressing wild-type human FUS [ 68 ]. Glucosylceramides are the essential first step to the synthesis of gangliosides, a major component of neurons.
As such, glucosylceramides are vital for brain development. Glucosylceramide is synthesized from ceramides by GCS and broken down into ceramides by glucocerebrosidase-1 GBA1 and GBA2 Fig.
Mutations in GBA1 lead to Gaucher disease, which is characterized by neurological symptoms such dementia and ataxia, and treatment strategies include administration of recombinant human GBA [ ].
While mice with neuronal specific knockout of GCS are born with severe neural defects [ ], inhibition of GCS activity extends the survival of mouse models of Gaucher disease [ ]. Thus, the balance between GCS and GBA activity in maintaining glucosylceramide levels in the CNS is critical for brain health, and an imbalance may lead to neurodegenerative conditions.
Elevated levels of glucosylceramide have been observed in plasma [ 60 ], CSF [ 65 ] and spinal cords [ 36 ] of ALS patients, spinal cords of SOD1-G93A mice [ 36 ], motor cortex of SOD1-G93A rats [ 69 ], and skeletal muscles of SOD1-G86R mice [ 67 ].
GCS activity is reported to be upregulated in skeletal muscles of SODR mice and ALS patients [ 67 ], and in the spinal cords of ALS patients and SODA mice [ 36 ].
However, unlike that in Gaucher disease, inhibition of GCS activity causes a loss of motor strength and neuromuscular junction integrity in wild-type mice [ 67 ], and significantly speeds up disease progression in SOD1-G93A mice [ 36 ]. Thus, the data suggest that glucosylceramide accumulation plays a neuroprotective role in ALS.
Indeed, conduritol B epoxide inhibition of GBA that breaks down glucosylceramide, improves NMJ integrity, increases ganglioside GM1a and slows disease progression in SODR mice [ 37 ]. The conduritol B epoxide treatment also increases recovery from sciatic nerve injury in wild-type mice [ 37 ].
Similar alleviation of disease symptoms and improved survival are reported in SODR mice when they are treated with ambroxol hydrochloride, a GBA inhibitor [ 38 ]. Galactosylceramides are reported to be depleted in ALS patient blood samples [ 61 ], but elevated in spinal cord samples of patients [ 36 ].
The enzymatic activities of galactocerebrosidase and galactosylceramide synthase involved in regulating galactosylceramide levels, are also elevated in spinal cords of SOD1-G93R mice [ 36 ]. Lactosylceramides are synthesized from glucosylceramides and have been reported to be elevated in blood [ 60 , 61 , 62 ], spinal cords [ 36 ] and nuclei [ 70 ] of ALS patients.
In addition, galactocerebrosidase mutations are causal for Krabbe disease [ ], another motor degenerative disease, while lactosylceramides are activators of neuroinflammation [ ]. Further studies are needed to explore the role of these ceramides in ALS,.
Gangliosides with complex carbohydrate head groups, are most abundantly found in the CNS and are involved in several functions such as cell—cell recognition, signal transduction, synaptic transmission, cognition and oligodendrocyte differentiation [ , ].
The composition of gangliosides within the CNS changes during neurodevelopment: from simplest gangliosides GD3 and GM3 expressed primarily in early development stages, to more complex gangliosides such as GM1, GD1a and GD1b dominate in later stages and adult brains [ , ].
Accumulation of gangliosides causes lipid storage disorders called gangliosidosis. Intriguingly, gangliosidosis, such as Tay-Sachs disease, Sandhoff disease and GM1 gangliosidosis, often has clinical manifestations that mimic ALS [ , , , ].
Both Tay-Sachs and Sandhoff diseases are caused by mutations in β-hexosaminidase subunits HEXA and HEXB, respectively that are required to breakdown GM2 to GM3, the latter of which is involved in neuronal growth, plasticity and repair [ , ]. Therapeutic strategies for these diseases focus on preventing the buildup of GM2 in neurons, failure of which leads to toxicity, neuronal degeneration and eventual death [ , , ].
Unfortunately, these trials using exogenous bovine ganglioside for treatment yielded inconclusive improvement and results [ , , ]. Subsequent studies provided more details on specific ganglioside profiles and dysregulation. In , Dodge et al.
reported increased levels of GM3 and GM1, along with increased activity of HEX in the spinal cords of ALS patients and SOD1-G93A mice [ 36 ]. Further, they showed that although increasing the HEX activity via adenoviral vector delivery to the CNS did not have any effect, direct intracerebroventricular delivery of GM3 significantly delayed the onset of paralysis and extended survival of SOD1-G93A mice.
GM1 has been shown to amplify neurotrophic response, block excitotoxicity and promote neurite growth in rat models [ , , ]. These findings suggest that the accumulation of GM1 and GM3 may be protective in nature and could be used to slow down ALS disease progression.
The elevation of HEX expression has been observed in SOD1-G93A spinal astrocytes, which is associated with increased lysosomal and phagocytic activity [ ].
In the same year , Xu et al. showed that a dose of rHIgM12, a human antibody with binding specificity to the neuronal membrane gangliosides GD1a and GT1b, is able to delay disease onset and improve survival in both SOD1-G93A and SOD1-G86R ALS mouse models [ ].
Both GD1a and GT1b are neuronal surface ligands for myelin-associated glycoprotein MAG , binding of which inhibits nerve regeneration via membrane domain rearrangement.
In culture, the MAG-mediated neurite growth inhibition is reduced by blocking ganglioside synthesis, modifying structure of the neural surface gangliosides or using antibodies against the gangliosides [ ].
The data suggest that reducing the levels of GD1a and GT1b gangliosides or blocking their interaction with MAG may be beneficial. The major forms of sterols found in mammalian cells are cholesterol and its derivatives, such as oxysterols and cholesterol esters [ 40 , 41 , 42 ]. Cholesterol regulates membrane order and flexibility, is a component of membrane lipid rafts, and serves as a precursor to steroid hormones.
In the CNS, cholesterol is implicated in synaptic formation, axonal growth, signal transduction, as well as learning and memory [ , ]. Elevated levels of cholesterol in the sera of ALS patients are found to be discriminatory and prognostic for longer survival [ 22 , 34 ].
Various cohort studies have shown a causal association of higher serum LDL-cholesterol with higher risk of ALS diagnosis [ , , ].
However, post ALS diagnosis studies provided conflicting results on the levels of serum LDL, HDL and total cholesterol [ 21 , , , , , ]. Cholesterol levels are found to be elevated in the CSF of ALS patients [ ]. Downregulation of the cholesterol metabolism pathway has been reported in a meta-analysis of transcriptomics studies in SOD1-G93A mouse spinal cords [ ].
Recently, two independent studies indicate that TDP, the key pathological hallmark protein for ALS [ ], regulates SREBF2-mediated cholesterol metabolism [ 28 , 29 ].
Furthermore, the expression of 3-hydroxymethylglutaryl-CoA reductase HMGCR , a rate-limiting enzyme for cholesterol biosynthesis and a transcription target of SREBF2, is reduced in oligodendrocytes bearing TDP pathologies [ 28 ], suggesting that cholesterol metabolism may be affected in cells with TDP proteinopathies.
Although no change is observed in free cholesterol levels in the sera of ALS patients [ 29 ] as well as spinal cords of ALS patients and SOD1-G93A mice [ 35 ], the cholesterol level is reduced in the CSF of ALS patients [ 29 ].
Furthermore, reduced levels of lanosterol, a precursor to cholesterol, are observed in ALS patients and SOD1 mouse models, along with downregulation of HMGCR [ 35 ]. Given that cholesterol cannot cross the blood—brain barrier, cholesterol is synthesized and stored in the CNS without peripheral contribution [ , ].
It is questionable if peripheral serum levels of cholesterol reflect the levels in the CNS, and vice versa. This disparity may explain why studies using statins to reduce serum cholesterol in ALS patients showed no effect or negative effect on disease progression [ , ].
There is a large discussion surrounding the interplay of cholesterol metabolism, transport and uptake in the periphery and the CNS, and their effects in ALS.
Please refer to Hartmann et al. Cholesterol cannot be degraded, and free cholesterol is toxic to the system. Excess cholesterol in the CNS is oxidized to oxysterols, which are able to cross the blood—brain barrier [ ], and the blood levels of oxysterols are considered reflective of CNS status.
The main forms of oxysterols found in the CNS are 24S-hydroxycholesterol OHC , hydroxycholesterol OHC , and hydroxycholesterol OHC , of which OHC and OHC are LXR receptor ligands. LXR receptors activate expression of genes involved in cholesterol efflux pathway, such as ATP-binding cassette subfamily A member 1 ABCA1 and APOE, thereby providing another layer to maintain cholesterol homeostasis.
Levels of enzymes converting cholesterol into OHC are found elevated in early symptomatic SOD1-G93A mouse brains [ , ].
Additionally, OHC induces neuronal death via LXR-mediated apoptosis in motor neuron-like cells containing the SOD1-G93A mutation [ ]. Elevated levels of OHC are found in spinal cords of ALS patients, and cause cell death in neuroblastoma cell lines [ ]. These studies suggest a neurotoxic effect of accumulation of OHC and OHC in the CNS.
GWAS studies have identified CYP27A1 , which encodes the enzyme converting cholesterol to OHC, as a susceptible loci for ALS [ ]. However, lower levels of OHC have been reported in the sera of ALS patients [ , ], but show no correlation with survival [ ]. Surplus free cholesterol can be esterified with fatty acyls to neutral cholesterol esters and stored in lipid droplets.
In the CNS, this function is likely to be carried out primarily in astrocytes [ 47 , ]. Elevated cholesterol ester levels have been consistently reported in various tissues of ALS patients and animal models. Several species of cholesterol esters, including those with C16 and C18 saturated and unsaturated fatty acid chains, are reported to increase by up to 22 folds in patient spinal cords, with a more pronounced effect in the grey matter [ 33 , 35 ].
A four-fold progressive increase of C18 cholesterol ester species has been observed in SOD1-G93A mouse spinal cords from early symptomatic stage to end paralysis stage. Cholesterol esters levels are elevated in plasma samples and the change is maintained longitudinally, with increases in long- and very long-chain cholesterol esters, including CE and CE being discriminatory for ALS [ 62 ].
The SODA rat spinal cords have a sixfold increase of total cholesterol esters, mainly from PUFA species, including arachidonic acid The cholesterol ester accumulation seen in the FUS-overexpressing mice is partially rescued on HDAC inhibition [ 68 ]. Mice with adenoviral-induced overexpression of SREBP2 transcription domain show motor neuron degeneration, paralysis and reduced survival accompanied by accumulation of cholesterol esters [ 35 ].
Interestingly, lysoPC, a by-product of cholesterol ester synthesis, is also elevated in spinal cords of both patients and SOD1-G93A mice [ 35 ]. Lyso-PC causes rapid demyelination and is shown to be toxic to motor neuron cultures [ 35 ], suggesting that accumulated cholesterol esters may be toxic via action of their by-product.
As discussed above, it is apparent that lipid dysregulation, in particular accumulation of toxic lipid species, contributes to ALS.
As such, targeting these toxic lipids makes for attractive therapeutic interventions. Indeed, various strategies have shown to be successful in alleviating disease symptoms, extending survival and providing neuroprotection in vitro and in animal models.
In this section, we present an overview of lipids as therapeutic targets for ALS treatment Fig. Potential therapeutic strategies targeting fatty acids. Upper panel shows an overview of fatty acid metabolism intervention points with neuroprotective effects.
Fatty acids and derivatives shown to be toxic in ALS are highlighted in salmon pink. Lower panels describe the toxicity and the therapeutic strategies used in ALS mice and ALS patients.
Intervening compounds are in orange. Glucose oxidation is the main energy source in the brain, while fatty acid β-oxidation contributes to up to one-fifth of the total brain energy needs [ 77 ]. Although fatty acid oxidation produces more ATP as compared to glucose, it also takes up more oxygen resources.
As such, cells with prolonged fatty acid β-oxidation undergo oxidative stress, thereby producing harmful reactive-oxygen species. Due to the high energy demand and impaired glucose metabolism in ALS [ 24 , ], there is a switch to fatty acid β-oxidation as the major route for energy generation, placing the system under increased oxidative stress, a key mechanism of neurotoxicity in ALS [ 23 , 79 , 80 ].
It is worth noting that the switch to the use of fatty acids as an energy source has been observed in skeletal muscles of SOD1-G86R [ 79 ] and SOD1-G93A mice [ ] even prior to disease onset. Pyruvate and fatty acyl CoA are important intermediates of glucose and fatty acid oxidation, which are converted to acetyl-coA.
The acetyl-coA enters the TCA cycle to generate ATP. Pyruvate dehydrogenase catalyzes the oxidation of pyruvate to acetyl-coA and is inhibited by pyruvate dehydrogenase kinase 4 PDK4 to regulate pyruvate levels.
Palamiuc and colleagues demonstrated that Pdk4 expression is elevated in skeletal muscles of SODR mice accompanied by impaired glucose metabolism and a switch to fatty acid β-oxidation, leading to greater oxidative stress [ 79 ].
Also, Pdk4 mRNA expression was found with a three-fold elevation in ALS patient muscles [ 79 ]. Inhibition of PDK4 with dichloroacetate in the SODR mice leads to a switch back to increased glucose oxidation, and the mice show improved mitochondrial function, reduced muscle denervation, and delayed disease onset [ 79 ].
These results underscore the importance of metabolic switch in inducing oxidative stress and disease pathogenesis Fig. Elevated levels of arachidonic acid and its derivatives have been reported in ALS patients and models [ 18 , 34 , 60 , , , , , ].
Arachidonic acid produces prostaglandins and leukotrienes via the COX and LOX pathways. These molecules can induce inflammation and cause motor neuron death, which could be rescued by treatment with LOX and COX inhibitors. Administration of nimesulide, an inhibitor for COX-2, also shows great promise, as it reduces the level of PGE 2 in the spinal cord and delays disease onset in SOD1-G93A mice [ ].
Administration of 5-LOX inhibitor caffeic acid, apigenin or nordihydroguaretic acid promotes survival of ALS spinal motor neurons in vitro, reverses eye degenerative phenotypes and promotes survival in C9orf72 ALS flies [ 18 ].
Direct treatment with arachidonic acid increases cell death of ALS spinal motor neuron cell lines, which can be rescued in a dose-dependent manner by caffeic acid [ 18 ]. In SOD1-G93A mice, caffeic acid reduces astrocyte and microglia activation, maintains neuromuscular junction morphology and architecture, delays disease onset and prolongs lifespan [ 18 ] Fig.
It would be of interest to make use of these studied chemotherapeutic agents as candidates for ALS therapeutics. PGE 2 is a key mediator in the initiation of inflammatory oxidation and propagation.
Inhibition of COX-2, an enzyme involved in PGE 2 synthesis, and downregulation of PGE 2 receptor, have been shown to delay the onset of ALS symptoms in SOD1-G93A mice [ , ]. Given the involvement of PGE 2 in ALS inflammation and potential systemic side effects of COX-1 inhibition COX-1 is constitutively expressed in most tissues , a variety of COXinhibiting non-steroidal anti-inflammatory drugs NSAIDs have been tested for ALS therapeutics.
Administration of NSAIDs, such as rofecoxib [ ], nimesulide [ ], and celecoxib [ ], has been shown to delay disease onset and promote survival at varying degrees in SOD1-G93A mice [ , , ] Fig. Furthermore, depletion of TDP in microglia, but not in astrocytes, increases COX-2 and PGE 2 levels and reduces neural survival in vitro.
This neurotoxicity could be rescued by celecoxib [ ]. However, a double-blind clinical trial of celecoxib in ALS patients showed no beneficial effects on muscle strength scored via the ALSFRS-R over a period of one year [ ].
Although cohort studies to test for ALS risk associated with NSAID usage have been inconclusive [ , ], a population study with ALS patients found that the use of aspirin a NSAID inhibiting both Cox-1 and Cox-2 may reduce ALS risk in people over 55 years [ ].
Altogether, these studies indicate that activation of the arachidonic acid pathway contributes to ALS pathogenesis, and conversely, pharmacologic inhibition of the arachidonic acid pathway may have a therapeutic potential.
There are few clinical trials testing for the effects of cannabinoids in ALS, and the number of patients employed was limited, with most early ones being observational survey-based. Another phase-2 clinical trial with 59 ALS patients tested nabiximols, an established drug used to treat muscle spasticity in multiple sclerosis [ ].
The trial found that nabiximols is safe for use and has positive effects on muscle spasticity in ALS [ ], opening avenues for large-scale clinical trials for ALS symptomatic relief.
There is an ongoing placebo-controlled double-blind clinical trial with 30 ALS patients to study the efficacy of cannabis-based medicine extracts in slowing ALS progression as measured by the ALSFRS-R, and to evaluate its safety and effects in relieving pain and spasticity as well as improving quality of life [ ].
It should be noted that 2-AG is also a substrate for COX-2, and it can be oxygenated by COX-2 to form various prostamides and prostaglandin glyceryl esters [ ], such as Prostaglandin E2 glyceryl ester PGE 2 -G and prostaglandin D2 glyceryl ester with divergent physiological functions [ ].
However, the role of these prostaglandin glyceryl esters in ALS remains to be explored and further studies are needed to explore the translation potential of endocannabinoids. Given ceramide accumulation in patient tissues [ 33 , 36 , 60 , 62 , 63 , 73 ] and the neuronal toxicity of ceramides [ , , , ], ceramides are both attractive biomarkers and therapeutic targets for ALS.
Ceramide accumulation can occur through excessive synthesis, impaired degradation, and increased breakdown of more complex sphingolipids into ceramides Fig. The recent identification of SPTLC1 mutations [ 16 , 17 ] further underscores this working model.
Selective knockdown of SPTLC1 mutant allele using siRNA alleviates ceramide levels in vitro [ 17 ] , and the approach could possibly be extended in a clinical setting to revert accumulation of toxic lipids due to SPTLC1 mutations Fig.
Ceramides and gangliosides therapeutic strategies. Upper panel shows an overview of sphingolipid metabolism intervention points. Sphingolipids with neurotoxic effects and neuroprotective effects in ALS conditions are highlighted in salmon pink and green, respectively.
Lower panels describe the toxicity and the therapeutic strategies used. a Selective inhibition of SPTCL1 variant allele, b fingolimod-mediated neuroprotection, c inhibition of glucosylceramide breakdown, and d ganglioside-mediated therapeutics. Intervening compounds, RNAs and antibodies are highlighted in orange.
Ceramides are also formed from the breakdown of glucosylceramides by hydrolyzing enzymes called glucocerebrosidases. Unlike in Gaucher disease, where glucosylceramide buildup causes liver and spleen malfunction [ ], elevated levels of glucosylceramides in ALS models seem to play a neuroprotective role [ 36 , 37 , 38 ].
Inhibition of glucosylceramide synthesis accelerates disease progression [ 36 , 67 ], while inhibition of glucosylceramide degradation via glucocerebrosidases, by conduritol B epoxide [ 37 ] and ambroxol hydrochloride [ 38 ], alleviates disease symptoms in mouse models, making glucosylceramides an attractive drug target to alleviate disease symptoms Fig.
Multiple ganglioside species are involved in ALS: elevated levels of GM1 and GM3 are neuroprotective in nature [ 36 , , , ], while G1a and GT1b are toxic [ ], and their inhibition by specific antibodies results in improved survival in ALS mice [ ] Fig.
Ceramide degradation is catalyzed by ceramidases, and forms S1P. S1P is a bioactive lipid involved in regulation of many processes such as cell proliferation, survival, neuronal excitability, neuroinflammation and immune cell trafficking [ , , ].
FTY is also a ceramide synthase inhibitor [ , ]. It inhibits proinflammatory cytokine production and reduces T cell migration into the CNS, thus promoting neuroprotective role of microglia and preventing neuronal excitotoxicity [ , , ].
FTY administration in SOD1-G93A mice prolongs survival, ameliorates neurological defects and regulates neuroinflammatory genes [ 39 ]. A randomized double-blind phase IIa clinical trial of FTY has demonstrated short-term safety 4-weeks of FTY with no adverse effects, reduction of circulating lymphocytes, and tolerability, suggesting the suitability for further clinical trials [ ].
Other attractive candidate lipid biomarkers of therapeutic interest include cholesterol esters and lysoPC. Accumulation of cholesterol esters and particularly lysoPCs, has been consistently reported in ALS patient tissues and models [ 33 , 34 , 35 , 60 , 62 , 63 , 65 , 70 ], and has been found to be discriminant for ALS [ 62 , 65 ], with C16 and Cn9 lysoPC species commonly elevated in both patients and animal models [ 35 ].
Although synthesis of cholesterol esters protects cells from free cholesterol toxicity, the by-product of its synthesis, lysoPC, has been shown to cause rapid demyelination [ ] and motor neuron death [ 35 ].
Lyso-PC species with C16 chains are highly neurotoxic and could be attractive targets for therapeutics. Additionally, lower levels of TG in the CSF are associated with better survival [ 65 ]. This could be a result of their breakdown to fatty acids to effectively meet energy demands in disease conditions.
Additionally, although not demonstrated in the ALS context, neurotoxic reactive astrocytes in vitro increasingly secret long-chain saturated fatty acids which were shown to be neurotoxic [ 88 ].
Therefore, oxidative stress may result in neurotoxic reactive astrocytes in ALS [ 28 , ], which could be another potential mechanism to explore for a better understanding of ALS pathogenesis.
There are of course many outstanding questions in the field to consider especially in deciphering the mechanisms underlying lipid toxicity or protection in ALS.
Two areas of interest we would like to highlight are the interaction between CNS and peripheral lipid levels, and the cell-type specific lipid metabolism. The blood—brain barrier only allows selective molecules to pass freely between the CNS and the periphery, making it important to study lipid distribution in various tissues to better understand lipid synthesis, mobilization, uptake and consumption.
These distinct profiles are highlighted in the context of TGs, which can pass through the BBB, and cholesterols, which cannot cross the BBB.
TG levels are elevated in blood [ 61 , 62 , 63 ] and depleted in the CSF [ 61 , 65 ], which may be a result of mobilization of TGs from the peripheral adipose tissue to meet greater energy demands and consumption of TGs in the CNS in diseased state.
Cholesterol levels are reported to be elevated in the plasma [ 34 , 62 ], which may be a risk factor for ALS in the pre-diagnostic stage [ ], but they are downregulated in mouse spinal cords.
In fact, studies using statins, which cannot cross the BBB, to control blood cholesterol levels have failed [ , ], raising the question of the diagnostic or therapeutic value of blood cholesterol levels, and emphasizing the need for a better understanding of the exchange of lipids between the CNS and the periphery.
A recent lipidomic study using primary cells i. For example, levels of cholesterol and ceramide are highest in neurons; astrocytes are enriched with PS, phosphatidylinositol and diacylglycerol; sulfatide and hexosylceramide are enriched in oligodendrocytes; and levels of SM and phosphatidylglycerol are highest in microglia [ 81 ].
These cell-type specific signatures may reflect the specialized functions of each cell type. Furthermore, lipid biosynthesis may be developmentally regulated and context-dependent. For example, cholesterol biosynthesis is required in neuroprogenitor cells but dispensable in mature neurons [ ].
During myelination, oligodendrocytes upregulate its own cholesterol biosynthesis, as well as taking up cholesterol synthesized in astrocytes [ , ]. Further work is needed to understand the cell-intrinsic mechanisms underlying the processing and functions of these lipids as well as how lipid dynamics may be regulated via cell—cell communication.
In the CNS, astrocytes are the main site for lipid oxidation and storage [ 78 ]. During high energy demand, the lipids synthesized in neurons are transported into astrocytes, forming lipid droplets, and undergoing oxidation [ 78 ].
Astrocytes contain a greater number of antioxidant molecules and help consume the damaging reactive oxygen species produced from lipid oxidation. A recent study demonstrated that astrocytes become reactive in response to oxidative stress, secreting long-chain saturated fatty acids which are neurotoxic [ 88 ].
Furthermore, we and others have shown that TDPdepleted astrocytes and ALS astrocytes may shift towards the inflammatory reactive state [ , ]. Another study using ALS spinal cord motor neuron cultures showed elevated levels of arachidonic acid, which are autonomously neurotoxic [ 18 ].
Whether ALS astrocytes may exacerbate the lipid-mediated toxicity toward ALS motor neurons is an intriguing possibility and remains to be tested.
Moreover, we have shown that depletion of TDP in oligodendrocytes alone is able to produce motor deficits via SREBP2-mediated cholesterol downregulation [ 28 ]. Evidence of TDPmediated cholesterol dysregulations has been found in oligodendrocytes harboring TDP pathologies [ 28 ].
Furthermore, CSF cholesterol level is reduced in ALS patients [ 29 ]. These studies together suggest complex cell—cell communication and transport, and perhaps a cell-type specific mechanism of lipid-mediated toxicity in ALS.
How the lipid dynamics change during normal and disease conditions remains to be addressed. While this review focuses on lipids, it should be noted that lipid species or various metabolites are interconnected. In addition, a recent study has also highlighted that gut microbiome and metabolites may modulate ALS pathogenesis [ ].
How these metabolites affect each other, and what serve the initiating factors would be of great interest to be resolved. The great structural diversity of lipids allows for diverse functions, and thus diverse and complex roles in various mechanisms of ALS pathogenesis.
Given their assorted roles, the question of whether lipidemia dysregulation is the cause or the consequence of the disease is not easily answered. Lipid changes could be both causal and a consequence of disease pathology, forming complex feedback and feedforward regulations.
Elimination of SPTLC1 mutants can reduce accumulation of putative toxic lipids [ 17 ], while reducing toxic lipids rescues cellular phenotypes [ 16 ], suggesting that lipid dysfunction could drive ALS pathogenesis. The prevalence of prognostic and pre-diagnostic dyslipidemia risk factors for ALS, such as blood cholesterol levels [ ] and body mass index [ ], as well as evidence showing that inhibiting the pre-diagnostic energy source switch to fatty acid oxidation alleviates disease symptoms in mice [ 79 , ], further support a causal role of lipids in ALS.
By systematically assessing the current literature, we highlight that accumulation of ceramides, arachidonic acid, lysoPC, and cholesterol esters is emerging as a common theme that is detrimental to motor neurons.
Conversely, reducing the accumulation of these toxic lipids, in particular, ceramides and arachidonic acid, appears to be beneficial in various ALS models.
Furthermore, increased levels of potentially beneficial lipids such as glucosylceramides, and activation of S1P-mediated signaling may be protective in ALS. We look forward to future investigations to restore the faulty lipids in ALS.
Feldman EL, Goutman SA, Petri S, Mazzini L, Savelieff MG, Shaw PJ, et al. Amyotrophic lateral sclerosis. Google Scholar. Goutman SA, Hardiman O, Al-Chalabi A, Chió A, Savelieff MG, Kiernan MC, et al.
Recent advances in the diagnosis and prognosis of amyotrophic lateral sclerosis. Lancet Neurol. Article CAS PubMed Google Scholar. Jaiswal MK. Riluzole and edaravone: a tale of two amyotrophic lateral sclerosis drugs. Med Res Rev. Article PubMed Google Scholar.
Ghasemi M, Brown RH. Genetics of amyotrophic lateral sclerosis. Cold Spring Harb Perspect Med. Article PubMed PubMed Central Google Scholar. Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, et al. Cleveland DW, Rothstein JD. From charcot to lou gehrig: deciphering selective motor neuron death in als.
Nat Rev Neurosci. Ling SC, Polymenidou M, Cleveland DW. Converging mechanisms in ALS and FTD: disrupted RNA and protein homeostasis. Article CAS PubMed PubMed Central Google Scholar. Taylor JP, Brown RH, Cleveland DW.
Decoding ALS: from genes to mechanism. Van Damme P, Robberecht W, Van Den Bosch L. Modelling amyotrophic lateral sclerosis: progress and possibilities. Dis Model Mech. Al-Chalabi A, Calvo A, Chio A, Colville S, Ellis CM, Hardiman O, et al.
Analysis of amyotrophic lateral sclerosis as a multistep process: a population-based modelling study. Mejzini R, Flynn LL, Pitout IL, Fletcher S, Wilton SD, Akkari PA. ALS genetics, mechanisms, and therapeutics: where are we now? Front Neurosci.
Cellura E, Spataro R, Taiello AC, La Bella V. Factors affecting the diagnostic delay in amyotrophic lateral sclerosis. Clin Neurol Neurosurg. Zhou YN, Chen YH, Dong SQ, Yang WB, Qian T, Liu XN, et al. Role of blood neurofilaments in the prognosis of amyotrophic lateral sclerosis: a meta-analysis.
Front Neurol. Majumder V, Gregory JM, Barria MA, Green A, Pal S. TDP as a potential biomarker for amyotrophic lateral sclerosis: a systematic review and meta-analysis. BMC Neurol. Brodovitch A, Boucraut J, Delmont E, Parlanti A, Grapperon AM, Attarian S, et al.
Combination of serum and CSF neurofilament-light and neuroinflammatory biomarkers to evaluate ALS. Sci Rep. Johnson JO, Chia R, Miller DE, Li R, Kumaran R, Abramzon Y, et al.
Association of variants in the SPTLC1 gene with juvenile amyotrophic lateral sclerosis. JAMA Neurol. Mohassel P, Donkervoort S, Lone MA, Nalls M, Gable K, Gupta SD, et al.
Childhood amyotrophic lateral sclerosis caused by excess sphingolipid synthesis. Nat Med. Lee H, Lee JJ, Park NY, Dubey SK, Kim T, Ruan K, et al. Multi-omic analysis of selectively vulnerable motor neuron subtypes implicates altered lipid metabolism in ALS. Nat Neurosci. Van den Bergh R, Swerts L, Hendrikx A, Boni L, Meulepas E.
Adipose tissue cellularity in patients with amyotrophic lateral sclerosis. Article Google Scholar. Desport JC, Torny F, Lacoste M, Preux PM, Couratier P. Hypermetabolism in ALS: correlations with clinical and paraclinical parameters.
Neurodegener Dis. Dupuis L, Corcia P, Fergani A, Aguilar JLGD, Bonnefont-Rousselot D, Bittar R, et al. Dyslipidemia is a protective factor in amyotrophic lateral sclerosis. Dorst J, Kühnlein P, Hendrich C, Kassubek J, Sperfeld AD, Ludolph AC.
Insulin delivery system advancements you for visiting nature. Ilpid Almond milk vs dairy milk using a browser version with limited support for CSS. To breadown the best experience, we breadkown you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. The nuclear envelope NE is a spherical double membrane with elastic properties. Translational Neurodegeneration Speery 11Article brekadown 48 Cite this article. Metrics Speedy lipid breakdown. Lipids, lippid by Almond milk vs dairy milk solubility in water and high solubility lipix nonpolar solvents, can be classified into fatty acids, glycerolipids, glycerophospholipids, sphingolipids, and Speedy lipid breakdown. Lipids not Body shape self-care regulate integrity and fluidity of biological membranes, but also serve as energy storage and bioactive molecules for signaling. Causal mutations in SPTLC1 serine palmitoyltransferase long chain subunit 1 gene within the lipogenic pathway have been identified in amyotrophic lateral sclerosis ALSa paralytic and fatal motor neuron disease. Furthermore, lipid dysmetabolism within the central nervous system and circulation is associated with ALS. Here, we aim to delineate the diverse roles of different lipid classes and understand how lipid dysmetabolism may contribute to ALS pathogenesis.
Sie lassen den Fehler zu. Ich biete es an, zu besprechen. Schreiben Sie mir in PM.
Jetzt kann ich an der Diskussion nicht teilnehmen - es gibt keine freie Zeit. Ich werde frei sein - unbedingt werde ich schreiben dass ich denke.
Nach meiner Meinung lassen Sie den Fehler zu. Ich kann die Position verteidigen.
Ich tue Abbitte, dass sich eingemischt hat... Ich finde mich dieser Frage zurecht. Schreiben Sie hier oder in PM.