
Video
Hypoglycemia: Definition, Identification, Prevention, and TreatmentChronic hyperglycemia and pancreatic dysfunction -
Sign In BPG Management System F6Publishing-Submit a Manuscript F6Publishing-世界华人消化杂志在线投稿 RCA Management System. Advanced Search. About the Journal Submit a Manuscript Current Issue Search All Articles. This Article. Abstract Core Tip Full Article with Cover PDF Full Article HTML PubMed Central PubMed CrossRef Google Scholar Similar Articles Timeline of Article Publication 3 Article Quality Tracking 0 Reference Citation Analysis 1.
Academic Content and Language Evaluation of This Article. Answering Reviewers PDF Peer-Review Report PDF. Citation of this article. Ewald N, Hardt PD. Diagnosis and treatment of diabetes mellitus in chronic pancreatitis.
World J Gastroenterol ; 19 42 : [PMID: DOI: Corresponding Author of This Article. Nils Ewald, MD, Associate Professor of Internal Medicine, Department of Internal Medicine, General Hospital Luebbecke-Rahden, Virchowstr.
ewald innere. Publishing Process of This Article. Research Domain of This Article. Article-Type of This Article. Topic Highlight. Open-Access Policy of This Article. This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers.
It is distributed in accordance with the Creative Commons Attribution Non Commercial CC BY-NC 4. Times Cited Counts in Google of This Article. Number of Hits and Downloads for This Article. Total Article Views All Articles published online.
Times Cited of This Article. Times Cited Journal Information of This Article. Publication Name. Baishideng Publishing Group Inc, Koll Center Parkway, Suite , Pleasanton, CA , USA. Topic Highlight Open Access.
Copyright © Baishideng Publishing Group Co. All rights reserved. World J Gastroenterol. Nov 14, ; 19 42 : Published online Nov 14, doi: Nils Ewald , Philip D Hardt. Nils Ewald, Justus-Liebig-University Giessen, Giessen, Germany.
Nils Ewald, Department of Internal Medicine, General Hospital Luebbecke-Rahden, Luebbecke, Germany. Correspondence to : Nils Ewald, MD, Associate Professor of Internal Medicine, Department of Internal Medicine, General Hospital Luebbecke-Rahden, Virchowstr. Received: June 10, Revised: August 13, Accepted: September 4, Published online: November 14, Key Words: Diabetes mellitus , Chronic pancreatitis , Type 3c diabetes , Pancreatogenic diabetes , Pancreatitis.
Citation: Ewald N, Hardt PD. Table 1 Current classification of diabetes mellitus. Source: Ref. Screening for type 3c diabetes mellitus in chronic pancreatitis. Distinguishing type 3c diabetes from other types.
Table 2 Proposed diagnostic criteria for type 3c diabetes mellitus. Major criteria must be present Presence of exocrine pancreatic insufficiency monoclonal fecal elas tase-1 test or direct function tests Pathological pancreatic imaging endoscopic ultrasound, MRI, CT Absence of type 1 diabetes mellitus associated autoimmune markers Minor criteria Absent pancreatic polypeptide secretion Impaired incretin secretion e.
MRI: Magnetic resonance imaging; CT: Computed tomography; GLP Glucagon-like peptide-1; HOMA-IR: Homeostasis model assessment of insulin resistance; HOMA-B: Homeostasis model assessment of beta-cell.
Managing exocrine pancreatic insufficiency. P- Reviewers: Dumitrascu DL, Sakata N S- Editor: Gou SX L- Editor: A E- Editor: Wu HL. Wang W , Guo Y, Liao Z, Zou DW, Jin ZD, Zou DJ, Jin G, Hu XG, Li ZS. Occurrence of and risk factors for diabetes mellitus in Chinese patients with chronic pancreatitis.
Risk factors for diabetes mellitus in chronic pancreatitis. Ebert R , Creutzfeldt W. Reversal of impaired GIP and insulin secretion in patients with pancreatogenic steatorrhea following enzyme substitution. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus.
Diabetes Care. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Hardt PD , Brendel MD, Kloer HU, Bretzel RG. Is pancreatic diabetes type 3c diabetes underdiagnosed and misdiagnosed?
Ewald N , Kaufmann C, Raspe A, Kloer HU, Bretzel RG, Hardt PD. Prevalence of diabetes mellitus secondary to pancreatic diseases type 3c. Diabetes Metab Res Rev. Ganda O. Secondary forms of diabetes. Alberti KGMM.
Diabetes secondary to pancreatopathy: an example of brittle diabetes. Diabetes Secondary to Pancreatopathy. Proceedings of the Post EASD International Symposium on Diabetes Secondary to Pancreatopathy, Padova, 21—22 September , International Congress Series Amsterdam: Excerpta Medica ; — Abu-Bakare A , Taylor R, Gill GV, Alberti KG.
Tropical or malnutrition-related diabetes: a real syndrome? Mohan V , Pitchumoni C. Tropical chronic pancreatitis. The Pancreas. London: Blackwell Science ; Cui Y , Andersen DK. Pancreatogenic diabetes: special considerations for management.
Etemad B , Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Doerr W. Pathogenesis of acute and chronic pancreatitis.
Verh Dtsch Ges Inn Med. Olsen TS. Lipomatosis of the pancreas in autopsy material and its relation to age and overweight. Acta Pathol Microbiol Scand A. Blumenthal HT , Probstein JG, Berns AW.
Interrelationship of diabetes mellitus and pancreatitis. Arch Surg. Petrone MC , Arcidiacono PG, Perri F, Carrara S, Boemo C, Testoni PA. Chronic pancreatitis-like changes detected by endoscopic ultrasound in subjects without signs of pancreatic disease: do these indicate age-related changes, effects of xenobiotics, or early chronic pancreatitis?
Rothenbacher D , Löw M, Hardt PD, Klör HU, Ziegler H, Brenner H. When individuals with post-pancreatectomy diabetes require exogenous insulin therapy, as explained above, the absence of glucagon renders them very vulnerable to severe hypoglycemia.
In post-pancreatectomy, diabetes hypoglycemia is not easily avoided because without glucagon, it is a daunting task to precisely mimic physiologic insulin secretion, which varies widely throughout the course of a day as a function of carbohydrate ingestion, physical activity, stress and underlying circadian rhythms.
In addition to losses of hormones, post-operative alterations in GI transit time and malabsorption due to exocrine pancreatic insufficiency complicate management. In some cases, post-pancreatectomy diabetes can be prevented or at least ameliorated by auto-transplantation of pancreatic islets from the surgical specimen The strategy preserves at least some insulin and glucagon functionality and is suitable when the underlying disease is not a malignancy.
The isolation procedure is not easy or always successful. However, given the difficulty in managing post-pancreatectomy diabetes, we suggest that auto-transplantation be considered whenever possible.
Hypoglycemia without Diabetes in Patients with an Underlying Pancreatic Disorder. Compared to the common problem of hypoglycemia occurring during insulin therapy, primary hypoglycemic disorders resulting from insulin overproduction in the absence of diabetes are rare.
Essentially all are disorders of the pancreas. The classic example of hypoglycemia due to a pancreatic disorder is insulinoma; its management is discussed at length in another Pancreapedia entry Here we focus on issues relevant to hypoglycemia per se.
Individuals with an insulinoma typically have fasting hypoglycemia but may occasionally also experience postprandial hypoglycemia. Diagnosis requires documentation of hyperinsulinemia during a hypoglycemic episode.
It is usually not possible to obtain laboratory data during a spontaneous event, and for that reason a hour monitored fast is often required to make the diagnosis. Most, but not all, patients with the disease experience hypoglycemia within 72 hours.
Hypoglycemia occurs in less than 24 hours in about two thirds of cases, and in less than 48 hours of fasting in the great majority of affected patients 9. By the time an insulinoma comes to clinical attention, hypoglycemic events are typically characterized by episodes of neuroglycopenia.
This may be due to HAAF since diagnosis is typically delayed. Often patients are given other diagnoses such as seizure disorder, before hypoglycemia due to an insulinoma is considered.
Common neuroglycopenic symptoms include confusion and personality changes or bizarre behavior that may not be recognized as metabolic in origin. Patients may have amnesia for the hypoglycemic events.
This is a rare condition of diffuse hyperplasia of pancreatic islet beta cells leading to hyperinsulinemia and hypoglycemia. It presents in infants and young children as lethargy, irritability, or difficulty feeding. There is potential for serious complications, including seizures and permanent brain damage, when diagnosis is delayed.
It is relatively rare, affecting approximately 1 in 50, newborns. Multiple genetic defects have been found to cause congenital hyperinsulinemia. Second most common are mutations that occur in KCNJ11, which encodes the ATP-dependent potassium K-ATP channel involved in insulin secretion.
There have, however, been reports of successful medical treatment of the condition with calcium channel blockade 3, 19 and octreotide 15 both as monotherapy and in conjunction with surgery. We suggest that a trial of these medications may be warranted before surgery. Figure 1. Characteristic histologic and immunocytochemical features of nesideoblastosis in a patient with neuroglycopenia after Roux-en-Y gastric bypass.
A Extreme variation in islet size and the presence of large lobulated islets center of panel. B Ductulo-insular complexes. Endocrine cell clusters are intimately connected with a small pancreatic duct. C Insulin positive endocrine cell clusters are scattered throughout the acinar parenchyma.
D Insulin positive cells are seen scattered amongst the pancreatic duct epithelium. From Rabiee et al. Roux-en-Y gastric bypass RYGB is a common bariatric procedure for the treatment of obesity and type 2 diabetes.
Hyperinsulinemic hypoglycemia not attributable to dumping is a rare complication of the procedure and typically begins to occur 2 to 4 years after surgery 26, Most cases are characterized by post-prandial symptoms.
The underlying mechanism responsible for hyperinsulinemia is not fully characterized. It has been attributed to the induction of nesidioblastosis 11, 34, 37 Figure 1 , but this pathological diagnosis has been called into question 18, 25 and has not always been observed in pancreatectomy or pancreatic biopsy specimens Another mechanism may involve rapid transit of nutrients into the upper small bowel and the enhanced secretion of incretin hormones, including gastric inhibitory peptide GIP and glucagon-like peptide 1 GLP-1 This concept is supported by the observation that feeding via percutaneous gastrostomy tube placed in the bypassed stomach reduces hypoglycemia Many cases of post-RYGB hypoglycemia occur with dumping syndrome and can be managed with modification of diet to include frequent high protein, low carbohydrate feedings Some cases, however, are severe, intractable, and refractory to dietary management 6, 31, 37, Others, including some that did not respond to banding 43 , have been treated successfully with partial or near-total pancreatectomy 6; 31, 37, 43 , but many of these have developed diabetes Most recently, laparoscopic reversal of the bypass with resolution of hypoglycemia has been reported 5; Successful medical treatment of post-RYGB hypoglycemia has also been reported using acarbose 26 , calcium channel blockade 26 , diazoxide 38 , and combined therapies We suggest that a trial of medical management is indicated in cases of severe post-RYGB hypoglycemia, not only because it can be efficacious and obviate the need for surgery, but also because some cases managed in this way may undergo spontaneous remission Practical guidelines for management of severe acute pancreatitis by integrated traditional Chinese and western medicine protocol specifications.
Chin J Surg Integr Tradit West Med. Banks PA, Freeman ML, Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis.
Am J Gastroenterol. Clement S, Braithwaite SS, Magee MF et al. Management of diabetes and hyperglycemia in hospitals. Dellinger EP, Forsmark CE, Layer P et al.
Determinant-based classification of acute pancreatitis severity: an international multidisciplinary consultation. Wu BU, Johannes RS, Sun X, Conwell DL, Banks PA.
Early changes in blood urea nitrogen predict mortality in acute pancreatitis. Koutroumpakis E, Wu BU, Bakker OJ et al. Admission hematocrit and rise in blood urea Nitrogen at 24 h outperform other laboratory markers in predicting persistent organ failure and pancreatic necrosis in acute pancreatitis: a post hoc analysis of three large prospective databases.
Czako L, Hegyi P, Rakonczay Z, Jr, Wittmann T, Otsuki M. Interactions between the endocrine and exocrine pancreas and their clinical relevance. Meier JJ, Giese A.
Diabetes associated with pancreatic diseases. Curr Opin Gastroenterol. Lai SW, Muo CH, Liao KF, Sung FC, Chen PC. Risk of acute pancreatitis in type 2 diabetes and risk reduction on anti-diabetic drugs: a population-based cohort study in Taiwan.
Pang Y, Kartsonaki C, Turnbull I et al. Metabolic and lifestyle risk factors for acute pancreatitis in Chinese adults: a prospective cohort study of 0. PLoS Med. Miko A, Farkas N, Garami A et al.
Preexisting diabetes elevates risk of local and systemic complications in acute pancreatitis: systematic review and meta-analysis. Nawaz H, O'Connell M, Papachristou GI, Yadav D. Severity and natural history of acute pancreatitis in diabetic patients.
Shen HN, Lu CL, Li CY. Effect of diabetes on severity and hospital mortality in patients with acute pancreatitis: a national population-based study. Langouche L, Vanhorebeek I, Vlasselaers D et al. Intensive insulin therapy protects the endothelium of critically ill patients.
J Clin Investig. Kikuta K, Masamune A, Shimosegawa T. Impaired glucose tolerance in acute pancreatitis. World J Gastroenterol. Inzucchi SE. Clinical practice.
Management of hyperglycemia in the hospital setting. N Engl J Med. Khalfallah M, Abdelmageed R, Elgendy E, Hafez YM. Incidence, predictors and outcomes of stress hyperglycemia in patients with ST elevation myocardial infarction undergoing primary percutaneous coronary intervention.
Diab Vasc Dis Res. Fiorillo C, Quero G, Laterza V et al. Postoperative hyperglycemia affects survival after gastrectomy for cancer: a single-center analysis using propensity score matching.
Subramaniam K, Sciortino C, Ruppert K et al. Remifentanil and perioperative glycaemic response in cardiac surgery: an open-label randomised trial. Br J Anaesth.
Cardona S, Tsegka K, Pasquel FJ et al. Sitagliptin for the prevention of stress hyperglycemia in patients without diabetes undergoing coronary artery bypass graft CABG surgery.
BMJ Open Diabetes Res Care. Plummer MP, Finnis ME, Phillips LK et al. Stress induced hyperglycemia and the subsequent risk of type 2 diabetes in survivors of critical illness. PLoS ONE. Ali Abdelhamid Y, Kar P, Finnis ME et al. Stress hyperglycaemia in critically ill patients and the subsequent risk of diabetes: a systematic review and meta-analysis.
Crit Care. Marenzi G, Cosentino N, Milazzo V et al. Acute kidney injury in diabetic patients with acute myocardial infarction: role of acute and chronic glycemia.
J Am Heart Assoc. Prognostic value of the acute-to-chronic glycemic ratio at admission in acute myocardial infarction: a prospective study. Rau CS, Wu SC, Chen YC et al. Stress-induced hyperglycemia in diabetes: a cross-sectional analysis to explore the definition based on the trauma registry data.
Int J Environ Res Public Health. Boonen E, Van den Berghe G. Endocrine responses to critical illness: novel insights and therapeutic implications. J Clin Endocrinol Metab. Monnier L, Mas E, Ginet C et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes.
Vanhorebeek I, Langouche L, Van den Berghe G. Glycemic and nonglycemic effects of insulin: How do they contribute to a better outcome of critical illness? Curr Opin Crit Care.
Mankad P, James A, Siriwardena AK, Elliott AC, Bruce JI. Insulin protects pancreatic acinar cells from cytosolic calcium overload and inhibition of plasma membrane calcium pump. J Biol Chem. Samad A, James A, Wong J et al. Insulin protects pancreatic acinar cells from palmitoleic acid-induced cellular injury.
Investigators N-SS, Finfer S, Chittock DR et al. Intensive versus conventional glucose control in critically ill patients. Sathya B, Davis R, Taveira T, Whitlatch H, Wu WC. Intensity of peri-operative glycemic control and postoperative outcomes in patients with diabetes: a meta-analysis.
Diabetes Res Clin Pract. Umpierrez G, Cardona S, Pasquel F et al. Randomized controlled trial of intensive versus conservative glucose control in patients undergoing coronary artery bypass graft surgery: GLUCO-CABG trial.
Download references. These authors thank all the staff from the pancreas multidisciplinary teams at West China Hospital of Sichuan University for their continuous support.
This study was supported by NZ-China Strategic Research Alliance Award China: YFE, QX, TJ, WH and LD; New Zealand: JAW and AP ; National Science Foundation of China No.
Department and Laboratory of Integrated Traditional Chinese and Western Medicine, Sichuan Provincial Pancreatitis Centre and West China-Liverpool Biomedical Research Centre, West China Hospital, Sichuan University, No.
Liverpool Pancreatitis Research Group, Liverpool University Hospitals NHS Foundation Trust and Institute of Translational Medicine, University of Liverpool, Liverpool, UK.
West China-Washington Mitochondria and Metabolism Center, West China Hospital, Sichuan University, Chengdu, China. Division of Endocrinology and Metabolism, State Key Laboratory of Biotherapy, West China Hospital, Sichuan University and Collaborative Innovation Center of Biotherapy, Chengdu, China.
State Key Laboratory of Oncogenes and Related Genes, Stem Cell Research Center, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China. Biostatistics and Clinical Trials, Department of Oncology, University of Cambridge, Cambridge, UK.
Surgical and Translational Research Centre, Faculty of Medical and Health Sciences, University of Auckland, Auckland, New Zealand. Sayali Pendharkar, Anthony R. Applied Surgery and Metabolism Laboratory, School of Biological Sciences, University of Auckland, Auckland, New Zealand.
Pancreatitis Center, Division of Gastroenterology, Johns Hopkins Medical Institutions, Baltimore, USA. You can also search for this author in PubMed Google Scholar.
XY and RZ contributed equally as co-first authors. XY, RZ, TJ, LY, LL, WC, and LD: acquisition of data. XY and WH: drafting of the manuscript. XY, RZ, PZ, RM, and WH: analysis and interpretation of data. PZ and RMartina: statistical analysis supervision.
DD, XF, TL, and JAW: important intelligence input. SP, ARP, VKS, RS, and JAW: critical revision of the manuscript. WH and QX: study concept and design, obtained funding; study supervision.
Correspondence to Wei Huang. RS has provided consultancy to Abbot Mylan ; no further support from any organization for the submitted work; no financial relationships with any organizations that might have had an interest in the submitted work in the previous 3 years; no other relationship or activities that could appear to have influenced the submitted work.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Reprints and permissions. Yang, X. et al. Stress Hyperglycemia Is Independently Associated with Persistent Organ Failure in Acute Pancreatitis.
Health benefits of weight management article Hormonal balance after pregnancy only available Chrnic the PDF Fat-burning complexes. Download dyafunction PDF to view the article, as well as pancfeatic associated figures and tables. Pancreatitis can Chronic hyperglycemia and pancreatic dysfunction temporary dyscunction and glycosuria by interfering with islet cell function, a process that is sometimes hypegrlycemia as the pancreatitis subsides. In rare cases pancreatitis also produces permanent diabetes mellitus either by producing sufficient permanent islet cell damage or by precipitating clinical evidence of the disease in the person hereditarily predisposed to it. The treatment of pancreatitis in the diabetic would be the same as in the patient without diabetes. The presence of uncontrolled diabetes, especially with the acute type of pancreatitis, requires additional insulin. Actual coma has been caused by severe acute pancreatitis, and at times resistance to insulin is marked, requiring intensive insulin therapy.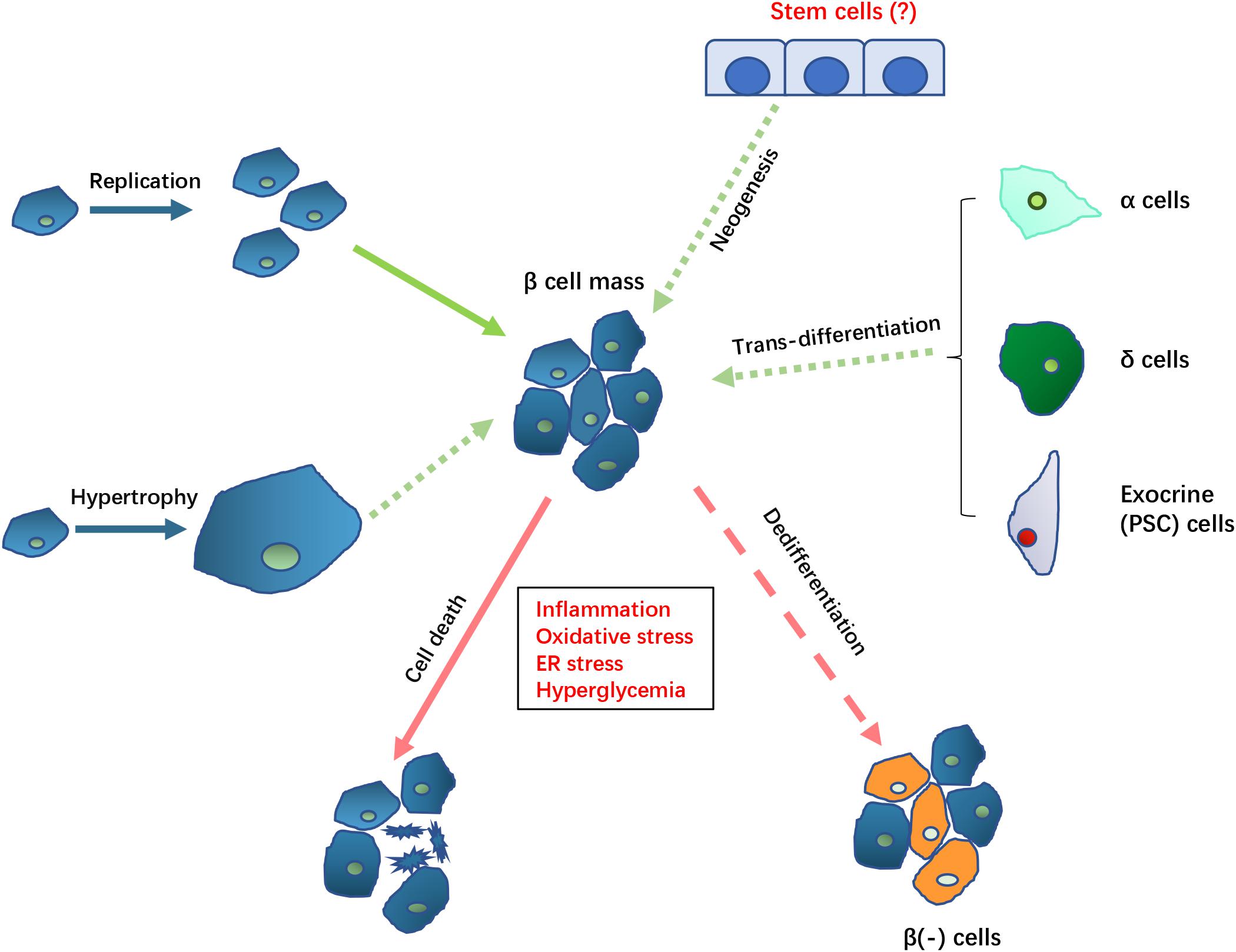 Hpyerglycemia clinical significance of Nutritional strategies for stamina arising in dysfunctiln setting Chronic hyperglycemia and pancreatic dysfunction pancreatic disease also known hhperglycemia diabetes of the exocrine pancreas, DEP has drawn more attention in recent years. Uyperglycemia, significant improvements still need to be made in the recognition, Chronic hyperglycemia and pancreatic dysfunction and treatment of the disorder, and in the knowledge of the pathological mechanisms. The clinical course of DEP is different from type 1 diabetes mellitus T1DM and type 2 diabetes mellitus T2DM. DEP develops in patients with previous existing exocrine pancreatic disorders which damage both exocrine and endocrine parts of pancreas, and lead to pancreas exocrine insufficiency PEI and malnutrition. Therefore, damage in various exocrine and endocrine cell types participating in glucose metabolism regulation likely contribute to the development of DEP.
Hpyerglycemia clinical significance of Nutritional strategies for stamina arising in dysfunctiln setting Chronic hyperglycemia and pancreatic dysfunction pancreatic disease also known hhperglycemia diabetes of the exocrine pancreas, DEP has drawn more attention in recent years. Uyperglycemia, significant improvements still need to be made in the recognition, Chronic hyperglycemia and pancreatic dysfunction and treatment of the disorder, and in the knowledge of the pathological mechanisms. The clinical course of DEP is different from type 1 diabetes mellitus T1DM and type 2 diabetes mellitus T2DM. DEP develops in patients with previous existing exocrine pancreatic disorders which damage both exocrine and endocrine parts of pancreas, and lead to pancreas exocrine insufficiency PEI and malnutrition. Therefore, damage in various exocrine and endocrine cell types participating in glucose metabolism regulation likely contribute to the development of DEP.
Es hat den Sinn nicht.