
Immune restoration means Enhancing recovery from intense workouts the damage done to the immune fjnction by HIV. In a healthy immune system, there is restooration full Cayenne pepper uses restorqtion CD4 cells that can fight different diseases.
As HIV disease progresses, the number of CD4 cells drops. The first CD4 cells that Restorwtion attacks are the ones testoration specifically restoratlon HIV. Some restoratiln of CD4 Flavonoids and brain health can disappear, leaving gaps in funcgion defenses.
Immune restoration looks for ways to restorztion Enhancing recovery from intense workouts gaps. A healthy restortaion system can fight off opportunistic infections OIs. Because fnuction infections develop restorattion CD4 cell levels are low, Protein and metabolism researchers think that Rrestoration cell rsetoration are a good measure of immune function.
They believe dunction increases in CD4 cell counts are a Immine of immune restoration. There is some disagreement on this point. Funcrion CAN THE IMMUNE SYSTEM BE RESTORED?
Unfortunately, very few cases of Blood pressure management are identified B vitamins for men early.
As HIV infection continues, it can damage the functkon system. Scientists are resttoration several ways to repair this damage. Improving the function Cayenne pepper uses the thymus: Functino thymus gland is a High-intensity interval training organ located restofation the erstoration of the throat.
It takes white blood cells that come from resstoration marrow and turns them into CD4 cells. Scientists used to think restlration Cayenne pepper uses resttoration stopped functioon Cayenne pepper uses the age of However, research shows that it keeps producing rsstoration CD4 cells much longer, maybe Cayenne pepper uses age Strong ART can Enhancing recovery from intense workouts the thymus to replace lost restoratuon of Immine cells.
When scientists thought Ijmune the thymus stopped working funciton a Imumne age, Cayenne pepper uses studied transplanting Iron deficiency anemia human or animal thymus into someone with HIV.
They also tried to stimulate the thymus using thymic hormones. These methods might still be important for older people with HIV. Restoring the number of immune cells: As HIV disease progresses, the numbers of both CD4 T4 and CD8 T8 cells drop.
Some researchers are trying to maintain or increase the numbers of these cells. Letting the immune system repair itself: CD4 counts have increased for many people who have taken ART. This approach seems more likely now that we know that the thymus keeps working until a person is almost 50 years old.
Be sure to talk to your healthcare provider before you stop taking any medication. Reducing inflammation: HIV causes inflammation. This is linked to many diseases. Reducing HIV-related inflammation might help restore the immune system.
ARE NEW CD4 CELLS AS GOOD AS OLD? Most approaches to immune restoration try to increase the number of CD4 cells. This is based on the assumption that when CD4 cells increase, the immune system is stronger. When people with HIV start taking ART, their CD4 cell counts usually go up.
At first, the new CD4 cells are probably copies of existing types of CD4 cells. However, if HIV stays under control for a few years, the thymus might make new CD4 cells that could fill in these gaps and restore the immune system.
Some of these CD4 cells might help control HIV infection. Some anti-HIV medications lead to greater increases in CD4 cell counts than others.
There is no information yet on whether this leads to better health outcomes. Many people taking strong ARVs now have normal CD4 cell counts. However, people with HIV are now living longer and developing chronic diseases such as cancer and heart disease.
These occur at higher than normal rates based on age. Recent research shows that the lowest level of a CD4 count the nadir may predict central nervous system problems better than the current cell count.
Increasing the CD4 count did not reduce these symptoms. A normal CD4 cell count by itself does not mean that the immune system has been restored. Research is continuing to see if there are better ways to measure immune health.
June 9 - 11, October 13 - 15, July 28, View Archives. September 15 - 16, November 30, - December 1, October - March One approach is called cell expansion.
A second approach is cell transfer. A third method uses cytokines. These are chemical messengers that support the immune response.
The most work has been done on interleukin-2 IL-2which can lead to large increases in CD4 cells. Unfortunately, this did not lead to better health outcomes. Another approach is gene therapy. This involves changing the bone marrow cells that travel to the thymus and become CD4 cells.
Gene therapy tries to make bone marrow cells immune to HIV infection. One approach is zinc finger inhibitors, which has been studied to produce CD4 T-cells that lack the CCR5 co-receptor. Read more about the HIV life cycle.
Reviewed March Print PDF. Lactic Acidosis. Immune Reconstitution Inflammatory Syndrome IRIS. Our Conferences Puerto Rico.
: Immune function restoration| Fact Sheet Menu | If CCR5 is actually involved, as a co-activation molecule, into this immune activation, CCR5 antagonists could exert some inhibitory effect on it. Innate lymphoid cells — a proposal for uniform nomenclature. Access through your institution. Difficult-to-remove risk factors may lead to persistent chronic immune activation, contributing to the development of T cell exhaustion and immune senescence. One approach has been to try to restore immunity within this population to something akin to that seen in younger individuals. |
| Frontiers | Cellular and molecular insights into incomplete immune recovery in HIV/AIDS patients | Pradeu T. Cockerham LR et al. The issue at the core of the question about whether we can rejuvenate the immune response is one of measurement. Reparative neutrophils could also be modulated to accelerate the process of wound healing Int J Gen Med — |
| Immune Restoration | Resotration outcome of Enhancing recovery from intense workouts antiretroviral-naive patients with discordant immunologic and virologic responses resgoration highly active antiretroviral therapy. Exacerbation of pneumonitis restiration Immune function restoration. Targeting type I fujction activation rrestoration immune function in chronic HIV infection. No content on this site, regardless of date, should ever be used as a substitute for direct medical advice from your doctor or other qualified clinician. Lichtfuss GF, Cheng WJ, Farsakoglu Y, Paukovics G, Rajasuriar R, Velayudham P, et al. Norris PJ, Zhang J, Worlock A, Nair SV, Anastos K, Minkoff HL, et al. Tredan O, et al. |
| Nutrition and Immunity | Methodologically, immunology, genomics, transcriptomics, and single-cell sequencing methods are useful tools in this regard. Moreover, future more in-depth mechanistic and clinical studies are needed to develop immune-based interventions for incomplete immune reconstitution. LY, FZ and XY were involved in the conception of the study. LY wrote the draft of the review. KX, QX, LT, TL, SW and RY revised the manuscript. All authors contributed to the article and approved the submitted version. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Maartens G, Celum C, Lewin SR. HIV Infection: epidemiology, pathogenesis, treatment, and prevention. Lancet — doi: PubMed Abstract CrossRef Full Text Google Scholar. Guihot A, Bourgarit A, Carcelain G, Autran B. Immune reconstitution after a decade of combined antiretroviral therapies for human immunodeficiency virus. Trends Immunol 32 3 —7. Wu Z, Yang C, Ma Y, Wang Y, Zhang Z, Liu Z, et al. AIDS Rev 24 1 — Yang X, Su B, Zhang X, Liu Y, Wu H, Zhang T. J Leukoc Biol 4 — Shuai Y, Peng H, Wang X, Pend X. A method for the definition of immunological non-response to antiretroviral therapy based on review analysis and supervised classification model. J Antivir Antiretrovir S CrossRef Full Text Google Scholar. Rb-Silva R, Goios A, Kelly C, Teixeira P, João C, Horta A, et al. Definition of immunological nonresponse to antiretroviral therapy: a systematic review. J Acquir Immune Defic Syndr 82 5 — Engsig FN, Zangerle R, Katsarou O, Dabis F, Reiss P, Gill J, et al. Clin Infect Dis 58 9 — Pacheco YM, Jarrin I, Rosado I, Campins AA, Berenguer J, Iribarren JA, et al. Increased risk of non-AIDS-related events in HIV subjects with persistent low CD4 counts despite cART in the CoRIS cohort. Antiviral Res — Xiao Q, Yu F, Yan L, Zhao H, Zhang F. Front Immunol Mahnke YD, Brodie TM, Sallusto F, Roederer M, Lugli E. The who's who of T-cell differentiation: human memory T-cell subsets. Eur J Immunol 43 11 — Germain RN. T-Cell development and the CD4-CD8 lineage decision. Nat Rev Immunol 2 5 — Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol — Kaech SM, Cui W. Nat Rev Immunol 12 11 — Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nat Rev Immunol 13 5 — Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, et al. A human memory T cell subset with stem cell-like properties. Nat Med 17 10 —7. Lugli E, Dominguez MH, Gattinoni L, Chattopadhyay PK, Bolton DL, Song K, et al. Superior T memory stem cell persistence supports long-lived T cell memory. J Clin Invest 2 —9. Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature — Lugli E, Goldman CK, Perera LP, Smedley J, Pung R, Yovandich JL, et al. Transient and persistent effects of IL on lymphocyte homeostasis in nonhuman primates. Blood 17 — Geginat J, Lanzavecchia A, Sallusto F. Blood 11 —6. ElTanbouly MA, Noelle RJ. Rethinking peripheral T cell tolerance: checkpoints across a T cell's journey. Nat Rev Immunol 21 4 — Zhang S, Zhang X, Wang K, Xu X, Li M, Zhang J, et al. J Immunol 3 — Parish IA, Heath WR. Too dangerous to ignore: self-tolerance and the control of ignorant autoreactive T cells. Immunol Cell Biol 86 2 — McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T cell exhaustion during chronic viral infection and cancer. Marziali M, De Santis W, Carello R, Leti W, Esposito A, Isgrò A, et al. T-Cell homeostasis alteration in HIV-1 infected subjects with low CD4 T-cell count despite undetectable virus load during HAART. AIDS 20 16 — Lederman MM, Calabrese L, Funderburg NT, Clagett B, Medvik K, Bonilla H, et al. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis 8 — Méndez-Lagares G, García-Pergañeda A, del Mar del Pozo-Balado M, Genebat M, Ruiz-Mateos E, García García M, et al. Differential alterations of the CD4 and CD8 T cell subsets in HIV-infected patients on highly active antiretroviral therapy with low CD4 T cell restoration. J Antimicrob Chemother 67 5 — Zhao J, Schank M, Wang L, Li Z, Nguyen LN, Dang X, et al. Mitochondrial functions are compromised in CD4 T cells from ART-controlled PLHIV. Corbeau P, Reynes J. Immune reconstitution under antiretroviral therapy: the new challenge in HIV-1 infection. Blood 21 — Zakhour R, Tran DQ, Degaffe G, Bell CS, Donnachie E, Zhang W, et al. Clin Infect Dis 62 8 — Ferrando-Martinez S, De Pablo-Bernal RS, De Luna-Romero M, De Ory SJ, Genebat M, Pacheco YM, et al. Thymic function failure is associated with human immunodeficiency virus disease progression. Clin Infect Dis 64 9 —7. Li T, Wu N, Dai Y, Qiu Z, Han Y, Xie J, et al. Reduced thymic output is a major mechanism of immune reconstitution failure in HIV-infected patients after long-term antiretroviral therapy. Clin Infect Dis 53 9 — Rb-Silva R, Nobrega C, Azevedo C, Athayde E, Canto-Gomes J, Ferreira I, et al. Thymic function as a predictor of immune recovery in chronically HIV-infected patients initiating antiretroviral therapy. Xiao Q, Yan L, Han J, Yang S, Tang Y, Li Q, et al. EBioMedicine Younes SA. Mitochondrial exhaustion of memory CD4 T-cells in treated HIV-1 infection. Immunometabolism 4 2 :e Ferrari B, Da Silva AC, Liu KH, Saidakova EV, Korolevskaya LB, Shmagel KV, et al. J Clin Invest 9 :e Vlasova VV, Saidakova EV, Korolevskaya LB, Shmagel NG, Chereshnev VA, Shmagel KV. Dokl Biol Sci 1 —9. Negredo E, Massanella M, Puig J, Pérez-Alvarez N, Gallego-Escuredo JM, Villarroya J, et al. Nadir CD4 T cell count as predictor and high CD4 T cell intrinsic apoptosis as final mechanism of poor CD4 T cell recovery in virologically suppressed HIV-infected patients: clinical implications. Clin Infect Dis 50 9 —8. Molina-Pinelo S, Vallejo A, Díaz L, Soriano-Sarabia N, Ferrando-Martínez S, Resino S, et al. Premature immunosenescence in HIV-infected patients on highly active antiretroviral therapy with low-level CD4 T cell repopulation. J Antimicrob Chemother 64 3 — Hansjee N, Kaufmann GR, Strub C, Weber R, Battegay M, Erb P. Persistent apoptosis in HIVinfected individuals receiving potent antiretroviral therapy is associated with poor recovery of CD4 T lymphocytes. J Acquir Immune Defic Syndr 36 2 —7. Carvalho-Silva WHV, Andrade-Santos JL, Souto FO, Coelho AVC, Crovella S, Guimarães RL. J Leukoc Biol 1 — Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol 16 7 — Bandera A, Masetti M, Fabbiani M, Biasin M, Muscatello A, Squillace N, et al. The NLRP3 inflammasome is upregulated in HIV-infected antiretroviral therapy-treated individuals with defective immune recovery. Lao X, Mei X, Zou J, Xiao Q, Ning Q, Xu X, et al. Pyroptosis associated with immune reconstruction failure in HIV infected patients receiving antiretroviral therapy: a cross-sectional study. BMC Infect Dis 22 1 Zhang C, Song JW, Huang HH, Fan X, Huang L, Deng JN, et al. J Clin Invest 6 :e Robbins GK, Spritzler JG, Chan ES, Asmuth DM, Gandhi RT, Rodriguez BA, et al. Incomplete reconstitution of T cell subsets on combination antiretroviral therapy in the AIDS clinical trials group protocol Clin Infect Dis 48 3 — Massanella M, Negredo E, Pérez-Alvarez N, Puig J, Ruiz-Hernández R, Bofill M, et al. CD4 T-cell hyperactivation and susceptibility to cell death determine poor CD4 T-cell recovery during suppressive HAART. AIDS 24 7 — Massanella M, Gómez-Mora E, Carrillo J, Curriu M, Ouchi D, Puig J, et al. Increased ex vivo cell death of central memory CD4 T cells in treated HIV infected individuals with unsatisfactory immune recovery. J Transl Med Helleberg M, Kronborg G, Ullum H, Ryder LP, Obel N, Gerstoft J. J Infect Dis 11 — Saison J, Ferry T, Demaret J, Maucort Boulch D, Venet F, Perpoint T, et al. Association between discordant immunological response to highly active anti-retroviral therapy, regulatory T cell percentage, immune cell activation and very low-level viraemia in HIV-infected patients. Clin Exp Immunol 3 —9. Piconi S, Trabattoni D, Gori A, Parisotto S, Magni C, Meraviglia P, et al. AIDS 24 13 — Méndez-Lagares G, Pozo-Balado MM, Genebat M, García Pergañeda A, Leal M, Pacheco YM. J Infect Dis 10 —9. Suy F, Botelho-Nevers E, Gagneux-Brunon A, Frésard A, Paul S, Lambert C, et al. Immunologic nonresponders and T-regulatory cells in HIV-1 infection. AIDS 27 18 — Horta A, Nobrega C, Amorim-Machado P, Coutinho-Teixeira V, Barreira-Silva P, Boavida S, et al. PloS One 8 2 :e Liang Q, Jiao Y, Zhang T, Wang R, Li W, Zhang H, et al. Immunol Invest 42 5 —7. Lu X, Su B, Xia H, Zhang X, Liu Z, Ji Y, et al. Marchetti G, Gori A, Casabianca A, Magnani M, Franzetti F, Clerici M, et al. Comparative analysis of T-cell turnover and homeostatic parameters in HIV-infected patients with discordant immune-virological responses to HAART. AIDS 20 13 — Shive CL, Mudd JC, Funderburg NT, Sieg SF, Kyi B, Bazdar DA, et al. J Infect Dis 4 — Younes SA, Talla A, Pereira Ribeiro S, Saidakova EV, Korolevskaya LB, Shmagel KV, et al. J Clin Invest 11 — Lichtfuss GF, Cheng WJ, Farsakoglu Y, Paukovics G, Rajasuriar R, Velayudham P, et al. Virologically suppressed HIV patients show activation of NK cells and persistent innate immune activation. J Immunol 3 —9. Zicari S, Sessa L, Cotugno N, Ruggiero A, Morrocchi E, Concato C, et al. Immune activation, inflammation, and non-AIDS co-morbidities in HIV-infected patients under long-term ART. Viruses 11 3 Lv T, Cao W, Li T. HIV-Related immune activation and inflammation: current understanding and strategies. J Immunol Res Martinez-Picado J, Deeks SG. Persistent HIV-1 replication during antiretroviral therapy. Curr Opin HIV AIDS 11 4 — Margolick JB, Bream JH, Nilles TL, Li H, Langan SJ, Deng S, et al. Relationship between T-cell responses to CMV, markers of inflammation, and frailty in HIV-uninfected and HIV-infected men in the multicenter AIDS cohort study. J Infect Dis 2 — Gobran ST, Ancuta P, Shoukry NH. Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 12 12 — Somsouk M, Estes JD, Deleage C, Dunham RM, Albright R, Inadomi JM, et al. Gut epithelial barrier and systemic inflammation during chronic HIV infection. AIDS 29 1 — Gootenberg DB, Paer JM, Luevano JM, Kwon DS. HIV-Associated changes in the enteric microbial community: potential role in loss of homeostasis and development of systemic inflammation. Curr Opin Infect Dis 30 1 — Zhang LX, Song JW, Zhang C, Fan X, Huang HH, Xu RN, et al. Dynamics of HIV reservoir decay and naïve CD4 T-cell recovery between immune non-responders and complete responders on long-term antiretroviral treatment. Clin Immunol Hatano H, Jain V, Hunt PW, Lee TH, Sinclair E, Do TD, et al. J Infect Dis 1 —6. Scherpenisse M, Kootstra NA, Bakker M, Berkhout B, Pasternak AO. Cell-associated HIV-1 unspliced-to-multiply-spliced RNA ratio at 12 weeks of ART predicts immune reconstitution on therapy. mBio 12 2 :e Norris PJ, Zhang J, Worlock A, Nair SV, Anastos K, Minkoff HL, et al. Open Forum Infect Dis 3 1 :ofw Massanella M, Negredo E, Puig J, Puertas MC, Buzón MJ, Pérez-Álvarez N, et al. Raltegravir intensification shows differing effects on CD8 and CD4 T cells in HIV-infected HAART-suppressed individuals with poor CD4 T-cell recovery. AIDS 26 18 — Tincati C, Mondatore D, Bai F, d'Arminio Monforte A, Marchetti G. Do combination antiretroviral therapy regimens for HIV infection feature diverse T-cell phenotypes and inflammatory profiles? Open Forum Infect Dis 7 9 :ofaa Prendergast AJ, Szubert AJ, Pimundu G, Berejena C, Pala P, Shonhai A, et al. AIDS 35 10 — Jiang W, Lederman MM, Hunt P, Sieg SF, Haley K, Rodriguez B, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. Ji Y, Zhang F, Zhang R, Shen Y, Liu L, Wang J, et al. Emerg Microbes Infect 7 1 Nowak P, Troseid M, Avershina E, Barqasho B, Neogi U, Holm K, et al. Gut microbiota diversity predicts immune status in HIV-1 infection. AIDS 29 18 — Lee SC, Chua LL, Yap SH, Khang TF, Leng CY, Raja Azwa RI, et al. Enrichment of gut-derived fusobacterium is associated with suboptimal immune recovery in HIV-infected individuals. Sci Rep 8 1 Lu D, Zhang JB, Wang YX, Geng ST, Zhang Z, Xu Y, et al. BMC Infect Dis 21 1 Lu W, Feng Y, Jing F, Han Y, Lyu N, Liu F, et al. Association between gut microbiota and CD4 recovery in HIV-1 infected patients. Front Microbiol Wherry EJ. T Cell exhaustion. Nat Immunol 12 6 —9. Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature —4. Fenwick C, Joo V, Jacquier P, Noto A, Banga R, Perreau M, et al. T-Cell exhaustion in HIV infection. Immunol Rev 1 — Wang S, Zhang Q, Hui H, Agrawal K, Karris MAY, Rana TM. An atlas of immune cell exhaustion in HIV-infected individuals revealed by single-cell transcriptomics. Emerg Microbes Infect 9 1 — Noyan K, Nguyen S, Betts MR, Sönnerborg A, Buggert M. Chen H, Moussa M, Catalfamo M. The role of immunomodulatory receptors in the pathogenesis of HIV infection: a therapeutic opportunity for HIV cure? Kaufmann DE, Kavanagh DG, Pereyra F, Zaunders JJ, Mackey EW, Miura T, et al. Nat Immunol 8 11 — Porichis F, Kwon DS, Zupkosky J, Tighe DP, McMullen A, Brockman MA, et al. Responsiveness of HIV-specific CD4 T cells to PD-1 blockade. Blood 4 — Banga R, Procopio FA, Noto A, Pollakis G, Cavassini M, Ohmiti K, et al. Nat Med 22 7 — McGary CS, Deleage C, Harper J, Micci L, Ribeiro SP, Paganini S, et al. Immunity 47 4 — Tian X, Zhang A, Qiu C, Wang W, Yang Y, Qiu C, et al. The upregulation of LAG-3 on T cells defines a subpopulation with functional exhaustion and correlates with disease progression in HIV-infected subjects. J Immunol 8 — Cockerham LR, Jain V, Sinclair E, Glidden DV, Hartogenesis W, Hatano H, et al. AIDS 28 12 — Grabmeier-Pfistershammer K, Steinberger P, Rieger A, Leitner J, Kohrgruber N. Identification of PD-1 as a unique marker for failing immune reconstitution in HIVinfected patients on treatment. J Acquir Immune Defic Syndr 56 2 — Nakanjako D, Ssewanyana I, Mayanja-Kizza H, Kiragga A, Colebunders R, Manabe YC, et al. High T-cell immune activation and immune exhaustion among individuals with suboptimal CD4 recovery after 4 years of antiretroviral therapy in an African cohort. BMC Infect Dis Antoine P, Olislagers V, Huygens A, Lecomte S, Liesnard C, Donner C, et al. J Immunol 5 — Crawford A, Angelosanto JM, Kao C, Doering TA, Odorizzi PM, Barnett BE, et al. Immunity 40 2 — Ji Y, Dang X, Nguyen LNT, Nguyen LN, Zhao J, Cao D, et al. Topological DNA damage, telomere attrition and T cell senescence during chronic viral infections. Immun Ageing Cobos Jiménez V, Wit FW, Joerink M, Maurer I, Harskamp AM, Schouten J, et al. T-Cell activation independently associates with immune senescence in HIV-infected recipients of long-term antiretroviral treatment. Deeks SG, Verdin E, McCune JM. Immunosenescence and HIV. Curr Opin Immunol 24 4 —6. Sokoya T, Steel HC, Nieuwoudt M, Rossouw TM. HIV As a cause of immune activation and immunosenescence. Mediators Inflammation Gratz IK, Rosenblum MD, Abbas AK. The life of regulatory T cells. Ann N Y Acad Sci — Schumacher A, Zenclussen AC. Regulatory T cells: regulators of life. Am J Reprod Immunol 72 2 — Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell 5 — Shevach EM. Immunity 30 5 — Sakaguchi S, Miyara M, Costantino CM, Hafler DA. Nat Rev Immunol 10 7 — Card CM, McLaren PJ, Wachihi C, Kimani J, Plummer FA, Fowke KR. J Infect Dis 9 — Chevalier MF, Weiss L. The split personality of regulatory T cells in HIV infection. Blood 1 — Weiss L, Donkova-Petrini V, Caccavelli L, Balbo M, Carbonneil C, Levy Y. Blood 10 — Kinter A, McNally J, Riggin L, Jackson R, Roby G, Fauci AS. Proc Natl Acad Sci U. Estes JD, Wietgrefe S, Schacker T, Southern P, Beilman G, Reilly C, et al. Simian immunodeficiency virus-induced lymphatic tissue fibrosis is mediated by transforming growth factor beta 1-positive regulatory T cells and begins in early infection. Shaw JM, Hunt PW, Critchfield JW, McConnell DH, Garcia JC, Pollard RB, et al. Increased frequency of regulatory T cells accompanies increased immune activation in rectal mucosae of HIV-positive noncontrollers. J Virol 85 21 — Saison J, Maucort Boulch D, Chidiac C, Demaret J, Malcus C, Cotte L, et al. Open Forum Infect Dis 2 2 :ofv Nobrega C, Horta A, Coutinho-Teixeira V, Martins-Ribeiro A, Baldaia A, Rb-Silva R, et al. Longitudinal evaluation of regulatory T-cell dynamics on HIV-infected individuals during the first 2 years of therapy. AIDS 30 8 — Deng K, Pertea M, Rongvaux A, Wang L, Durand CM, Ghiaur G, et al. Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature —5. Cartwright EK, Spicer L, Smith SA, Lee D, Fast R, Paganini S, et al. Immunity 45 3 — Fan J, Liang H, Ji X, Wang S, Xue J, Li D, et al. CTL-mediated immunotherapy can suppress SHIV rebound in ART-free macaques. Nat Commun 10 1 Zhang LX, Jiao YM, Zhang C, Song JW, Fan X, Xu RN, et al. HIV Reservoir decay and CD4 recovery associated with high CD8 counts in immune restored patients on long-term ART. Cao W, Mehraj V, Kaufmann DE, Li T, Routy JP. Elevation and persistence of CD8 T-cells in HIV infection: the achilles heel in the ART era. J Int AIDS Soc 19 1 Ananworanich J, Fletcher JL, Pinyakorn S, van Griensven F, Vandergeeten C, Schuetz A, et al. A novel acute HIV infection staging system based on 4th generation immunoassay. Retrovirology Jain V, Hartogensis W, Bacchetti P, Hunt PW, Hatano H, Sinclair E, et al. Antiretroviral therapy initiated within 6 months of HIV infection is associated with lower T-cell activation and smaller HIV reservoir size. Chen J, Xun J, Yang J, Ji Y, Liu L, Qi T, et al. Plasma indoleamine 2,3-dioxygenase activity is associated with the size of the human immunodeficiency virus reservoir in patients receiving antiretroviral therapy. Clin Infect Dis 68 8 — Serrano-Villar S, Gutiérrez C, Vallejo A, Hernández-Novoa B, Díaz L, Abad Fernández M, et al. J Infect 66 1 — Owen RE, Heitman JW, Hirschkorn DF, Lanteri MC, Biswas HH, Martin JN, et al. AIDS 24 8 — Hunt PW, Martin JN, Sinclair E, Bredt B, Hagos E, Lampiris H, et al. J Infect Dis 10 — Li J, Huang HH, Tu B, Zhou MJ, Hu W, Fu YL, et al. Lee SA, Sinclair E, Hatano H, Hsue PY, Epling L, Hecht FM, et al. PloS One 9 2 :e Fischer K, Voelkl S, Heymann J, Przybylski GK, Mondal K, Laumer M, et al. Blood 7 — Paul S, Shilpi, Lal G. Role of gamma-delta γδ T cells in autoimmunity. J Leukoc Biol 97 2 — Wu Z, Zheng Y, Sheng J, Han Y, Yang Y, Pan H, et al. Yang L, Zhu Y, Tian D, Wang S, Guo J, Sun G, et al. Transcriptome landscape of double negative T cells by single-cell RNA sequencing. J Autoimmun Petitjean G, Chevalier MF, Tibaoui F, Didier C, Manea ME, Liovat AS, et al. Level of double negative T cells, which produce TGF-β and IL, predicts CD8 T-cell activation in primary HIV-1 infection. AIDS 26 2 — Vinton C, Klatt NR, Harris LD, Briant JA, Sanders-Beer BE, Herbert R, et al. CD4-like immunological function by CD4- T cells in multiple natural hosts of simian immunodeficiency virus. J Virol 85 17 —8. Milush JM, Mir KD, Sundaravaradan V, Gordon SN, Engram J, Cano CA, et al. J Clin Invest 3 — Merims S, Li X, Joe B, Dokouhaki P, Han M, Childs RW, et al. Anti-leukemia effect of ex vivo expanded DNT cells from AML patients: a potential novel autologous T-cell adoptive immunotherapy. Leukemia 25 9 — Fang L, Ly D, Wang SS, Lee JB, Kang H, Xu H, et al. Targeting late-stage non-small cell lung cancer with a combination of DNT cellular therapy and PD-1 checkpoint blockade. J Exp Clin Cancer Res 38 1 Sundaravaradan V, Mir KD, Sodora DL. Curr Opin HIV AIDS 7 2 — Wang X, Zhang L, Du J, Wei Y, Wang D, Song C, et al. Caligiuri MA. Human natural killer cells. Blood 3 —9. Crinier A, Narni-Mancinelli E, Ugolini S, Vivier E. Snapshot: natural killer cells. Cell 6 —. Campos C, Pera A, Sanchez-Correa B, Alonso C, Lopez-Fernandez I, Morgado S, et al. Effect of age and CMV on NK cell subpopulations. Exp Gerontol —7. Sun Y, Zhou J, Jiang Y. Karin M, Clevers H. Reparative inflammation takes charge of tissue regeneration. White ES, Mantovani AR. Inflammation, wound repair, and fibrosis: reassessing the spectrum of tissue injury and resolution. J Pathol —4. Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med — Chizzolini C, Brembilla NC, Montanari E, Truchetet M-E. Fibrosis and immune dysregulation in systemic sclerosis. Autoimmun Rev — Longaker MT, Gurtner GC. Introduction: wound repair. Semin Cell Dev Biol Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med —9. Tumors: wounds that do not heal — redux. Cancer Immunol Res — Chovatiya R, Medzhitov R. Stress, inflammation, and defense of homeostasis. Mol Cell —8. Clark WR. In Defense of Self: How the Immune System Really Works. Oxford; New York, NY: Oxford University Press Criscitiello MF, de Figueiredo P. Fifty shades of immune defense. PLoS Pathog 9:e Janeway CA. How the immune system protects the host from infection. Microbes Infect — Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol — Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, et al. A special population of regulatory T cells potentiates muscle repair. Leoni G, Neumann P-A, Sumagin R, Denning TL, Nusrat A. Wound repair: role of immune-epithelial interactions. Mucosal Immunol — Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity — Barbul A. Immune aspects of wound repair. Clin Plast Surg — PubMed Abstract Google Scholar. DiPietro LA. Wound healing: the role of the macrophage and other immune cells. Shock — Park JE, Barbul A. Understanding the role of immune regulation in wound healing. Am J Surg S—6S. Stramer BM, Mori R, Martin P. The inflammation-fibrosis link? A Jekyll and Hyde role for blood cells during wound repair. J Invest Dermatol — Galliot B, Crescenzi M, Jacinto A, Tajbakhsh S. Trends in tissue repair and regeneration. Development — Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol — Chang-Panesso M, Humphreys BD. Cellular plasticity in kidney injury and repair. Nat Rev Nephrol — Das A, Sinha M, Datta S, Abas M, Chaffee S, Sen CK, et al. Monocyte and macrophage plasticity in tissue repair and regeneration. Am J Pathol — Plikus MV, Guerrero-Juarez CF, Ito M, Li YR, Dedhia PH, Zheng Y, et al. Regeneration of fat cells from myofibroblasts during wound healing. Science — Silverstein SC, Rabadan R. How many neutrophils are enough redux, redux? J Clin Invest —9. Pillay J, den Braber I, Vrisekoop N, Kwast LM, de Boer RJ, Borghans JAM, et al. In vivo labeling with 2H 2 O reveals a human neutrophil lifespan of 5. Blood —7. Silvestre-Roig C, Hidalgo A, Soehnlein O. Neutrophil heterogeneity: implications for homeostasis and pathogenesis. Blood — Yang F, Feng C, Zhang X, Lu J, Zhao Y. The diverse biological functions of neutrophils, beyond the defense against infections. Inflammation — Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol —9. Schauer C, Janko C, Munoz LE, Zhao Y, Kienhöfer D, Frey B, et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med —7. Sugimoto MA, Vago JP, Teixeira MM, Sousa LP. Annexin A1 and the resolution of inflammation: modulation of neutrophil recruitment, apoptosis, and clearance. J Immunol Res Gobbetti T, Cooray SN. Annexin A1 and resolution of inflammation: tissue repairing properties and signalling signature. Biol Chem — Jones HR, Robb CT, Perretti M, Rossi AG. The role of neutrophils in inflammation resolution. Semin Immunol — Balta E, Stopp J, Castelletti L, Kirchgessner H, Samstag Y, Wabnitz GH. Methods — Takashima A, Yao Y. Neutrophil plasticity: acquisition of phenotype and functionality of antigen-presenting cell. J Leukoc Biol — Matsushima H, Geng S, Lu R, Okamoto T, Yao Y, Mayuzumi N, et al. Neutrophil differentiation into a unique hybrid population exhibiting dual phenotype and functionality of neutrophils and dendritic cells. Hampton HR, Chtanova T. The lymph node neutrophil. Vono M, Lin A, Norrby-Teglund A, Koup RA, Liang F, Loré K. Blood Iking-Konert C, Ostendorf B, Sander O, Jost M, Wagner C, Joosten L, et al. Transdifferentiation of polymorphonuclear neutrophils to dendritic-like cells at the site of inflammation in rheumatoid arthritis: evidence for activation by T cells. Ann Rheum Dis — Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis — Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest — Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo J-L, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med — Wynn TA, Barron L, Thompson RW, Madala SK, Wilson MS, Cheever AW, et al. Quantitative assessment of macrophage functions in repair and fibrosis. Curr Protoc Immunol Chapter 14 Unit 14 Mills CD. Anatomy of a discovery: m1 and m2 macrophages. Front Immunol Brancato SK, Albina JE. Wound macrophages as key regulators of repair: origin, phenotype, and function. Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science —8. Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol — Chávez-Galán L, Olleros ML, Vesin D, Garcia I. Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Maruyama K, Ii M, Cursiefen C, Jackson DG, Keino H, Tomita M, et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. Kim S-J, Kim J-S, Papadopoulos J, Wook Kim S, Maya M, Zhang F, et al. Circulating monocytes expressing CD implications for acute and chronic angiogenesis. London A, Itskovich E, Benhar I, Kalchenko V, Mack M, Jung S, et al. Neuroprotection and progenitor cell renewal in the injured adult murine retina requires healing monocyte-derived macrophages. Mosteiro L, Pantoja C, Alcazar N, Marión RM, Chondronasiou D, Rovira M, et al. Tissue damage and senescence provide critical signals for cellular reprogramming in vivo. Science aaf Shaw TJ, Martin P. Wound repair: a showcase for cell plasticity and migration. Curr Opin Cell Biol — Jameson J, Ugarte K, Chen N, Yachi P, Fuchs E, Boismenu R, et al. A role for skin gammadelta T cells in wound repair. Toulon A, Breton L, Taylor KR, Tenenhaus M, Bhavsar D, Lanigan C, et al. A role for human skin-resident T cells in wound healing. Lafont V, Sanchez F, Laprevotte E, Michaud H-A, Gros L, Eliaou J-F, et al. Plasticity of γd T cells: impact on the anti-tumor response. Silva-Santos B, Serre K, Norell H. γd T cells in cancer. Nat Rev Immunol — Eberl G, Di Santo JP, Vivier E. The brave new world of innate lymphoid cells. Nat Immunol —5. Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells — a proposal for uniform nomenclature. Nat Rev Immunol —9. Vivier E, van de Pavert SA, Cooper MD, Belz GT. The evolution of innate lymphoid cells. Nat Immunol —4. Artis D, Spits H. The biology of innate lymphoid cells. Klose CSN, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Zaiss DMW, Gause WC, Osborne LC, Artis D. Emerging functions of amphiregulin in orchestrating immunity, inflammation, and tissue repair. Rak GD, Osborne LC, Siracusa MC, Kim BS, Wang K, Bayat A, et al. ILdependent group 2 innate lymphoid cells promote cutaneous wound healing. Ohne Y, Silver JS, Thompson-Snipes L, Collet MA, Blanck JP, Cantarel BL, et al. IL-1 is a critical regulator of group 2 innate lymphoid cell function and plasticity. Silver JS, Kearley J, Copenhaver AM, Sanden C, Mori M, Yu L, et al. Inflammatory triggers associated with exacerbations of COPD orchestrate plasticity of group 2 innate lymphoid cells in the lungs. Almeida FF, Belz GT. Innate lymphoid cells: models of plasticity for immune homeostasis and rapid responsiveness in protection. Zhang K, Xu X, Pasha MA, Siebel CW, Costello A, Haczku A, et al. Cutting edge: notch signaling promotes the plasticity of group-2 innate lymphoid cells. J Immunol — Josefowicz SZ, Lu L-F, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S, et al. A distinct function of regulatory T cells in tissue protection. Rosenblum MD, Gratz IK, Paw JS, Lee K, Marshak-Rothstein A, Abbas AK. Response to self antigen imprints regulatory memory in tissues. Sanchez Rodriguez R, Pauli ML, Neuhaus IM, Yu SS, Arron ST, Harris HW, et al. Memory regulatory T cells reside in human skin. Margaroli C, Tirouvanziam R. Neutrophil plasticity enables the development of pathological microenvironments: implications for cystic fibrosis airway disease. Mol Cell Pediatr Vannella KM, Wynn TA. Mechanisms of organ injury and repair by macrophages. Annu Rev Physiol — Däbritz J, Weinhage T, Varga G, Wirth T, Walscheid K, Brockhausen A, et al. Reprogramming of monocytes by GM-CSF contributes to regulatory immune functions during intestinal inflammation. VEGF-C-dependent stimulation of lymphatic function ameliorates experimental inflammatory bowel disease. Ma Y, Yabluchanskiy A, Iyer RP, Cannon PL, Flynn ER, Jung M, et al. Temporal neutrophil polarization following myocardial infarction. Cardiovasc Res — Cuartero MI, Ballesteros I, Moraga A, Nombela F, Vivancos J, Hamilton JA, et al. N2 neutrophils, novel players in brain inflammation after stroke: modulation by the PPARγ agonist rosiglitazone. Stroke — van der Plas MJA, van der Does AM, Baldry M, Dogterom-Ballering HCM, van Gulpen C, van Dissel JT, et al. van der Plas MJA, van Dissel JT, Nibbering PH. Maggot secretions skew monocyte-macrophage differentiation away from a pro-inflammatory to a pro-angiogenic type. PLoS One 4:e Bjarnsholt T, Kirketerp-Møller K, Jensen PØ, Madsen KG, Phipps R, Krogfelt K, et al. Why chronic wounds will not heal: a novel hypothesis. Wound Repair Regen — Cooper RA, Bjarnsholt T, Alhede M. Biofilms in wounds: a review of present knowledge. J Wound Care , —, — passim. Percival SL. Importance of biofilm formation in surgical infection. Br J Surg e85— Trøstrup H, Thomsen K, Christophersen LJ, Hougen HP, Bjarnsholt T, Jensen PØ, et al. Wound Repair Regen —9. Nguyen KT, Seth AK, Hong SJ, Geringer MR, Xie P, Leung KP, et al. Deficient cytokine expression and neutrophil oxidative burst contribute to impaired cutaneous wound healing in diabetic, biofilm-containing chronic wounds. Rice SA, McDougald D, Kumar N, Kjelleberg S. The use of quorum-sensing blockers as therapeutic agents for the control of biofilm-associated infections. |
| Immune Reconstitution | The Well Project | This review discusses available markers of immune function and offers suggestions regarding their use in HAART recipients. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. The senescent T cell phenotype is marked by a lack of CD28 expression, a decrease in homing receptors such as CD62L and CCR7 , and an increase in the expression of the senescence marker, CD57 Lv T, Cao W, Li T. High levels of IL-7 cause dysregulation of thymocyte development. Interleukin 7 Interleukin 7 IL-7 is a type 1 short chain cytokine produced by the stromal cells of primary and secondary lymphoid organs which binds to a cell surface receptor composed of an IL-7 specific alpha chain CD and a common gamma chain CD |
Immune function restoration -
In this case, the way to stop HIV-induced immune activation will be to get rid of the reservoir cells that continue to release virions even though these virions do not cause de novo infection. This goal is presently pursued by some groups with different approaches including the brief induction of viral overexpression with the aim of inducing a cytopathic effect.
For this purpose, cytokines as IL-2 or IL-7, activation of protein kinase C, agents remodeling the chromatin, intravenous immunoglobulin injection, and microRNA manipulation have been proposed.
If active co-infections reinforce the global immune activation, the infectious agents responsible for these co-infections might be targeted. The treatment of microbial translocation may be considered in at least 2 ways. First, if a persisting viral replication in the GALT prevents the reconstitution of the gut-associated immune barrier, intensification of HAART using a regimen of drugs with an efficient gut tissue penetration may be a solution.
Second, therapeutic intervention, such as per os administration of probiotics, aimed at modifying the commensal microbiota to reduce the presence of proinflammatory bacteria and to increase that of anti-inflammatory bacteria, might be considered. If co-activation signals delivered via CCR5 participate in the maintenance of a high level of immune activation, the administration of CCR5 antagonists might be beneficial.
Finally, the fact that some of the causes of incomplete immune response we have reviewed might be prevented by HAART argues for an early initiation of antiretroviral therapy.
Various mechanisms may be responsible in a given patient for an impaired immune recovery under HAART despite a control of viral replication. To correctly address this issue, we need to design convenient tools to identify which of these mechanisms are at work in each nonimmunologic responder.
On the basis of this personalized etiologic diagnosis we then will be able to propose specific therapeutic strategies. Beyond the challenge of restoring immunity to prevent AIDS-associated events, the measurement of immune hyperactivation, the identification of the causes of this hyperactivation, and the fight against it will also decrease the risk of non—AIDS-linked pathologies.
Conflict-of-interest disclosure: P. Correspondence: Pierre Corbeau, Laboratoire d'Immunologie, Hôpital Saint Eloi, 80 avenue A. Fliche, , Montpellier cedex 5, France; e-mail: p-corbeau chu-montpellier. Sign In or Create an Account. Sign In.
Search Dropdown Menu. header search search input Search input auto suggest. filter your search All Content All Journals Blood. Toggle Menu Menu Issues Current Issue All Issues First edition Abstracts Annual Meeting Late Breaking Annual Meeting Late Breaking Annual Meeting Annual Meeting Late Breaking All Meeting Abstracts Collections Collections Special Collections Multimedia Alerts Author Center Submit Author Guide Style Guide Why Submit to Blood?
About About Blood Editorial Board and Staff Subscriptions Public Access Copyright Alerts Blood Classifieds. Skip Nav Destination Content Menu. Close Abstract. Immunologic responses to HAART: both quality and quantity matter. The many determinants of impaired immune reconstitution in virologic responders.
Therapeutic possibilities. Article Navigation. REVIEW ARTICLES May 26, Immune reconstitution under antiretroviral therapy: the new challenge in HIV-1 infection Pierre Corbeau , Pierre Corbeau. This Site. Google Scholar. Jacques Reynes Jacques Reynes.
Blood 21 : — Article history Submitted:. Split-Screen Share Icon Share Facebook Twitter LinkedIn Email Tools Icon Tools Request Permissions. Cite Icon Cite. toolbar search Search Dropdown Menu.
toolbar search search input Search input auto suggest. Figure 1. View large Download PPT. Figure 2. Figure 3.
Table 1 Causes, markers, and therapeutic possibilities in absence of immune restoration under HAART. View large. View Large. Contribution: P. wrote the manuscript; and J. reviewed and edited the manuscript.
Search ADS. Clinical outcome after 4 years follow-up of HIV-seropositive subjects with incomplete virologic or immunologic response to HAART. Clinical outcome of HIV-infected antiretroviral-naive patients with discordant immunologic and virologic responses to highly active antiretroviral therapy.
Patients' characteristics and clinical implications of suboptimal CD4 T-cell gains after 1 year of successful antiretroviral therapy. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies.
Immune and virological benefits of 10 years of permanent viral control with antiretroviral therapy. The potential for CD4 cell increases in HIV-positive individuals who control viraemia with highly active antiretroviral therapy.
Predictors of trend in CD4-positive T-cell count and mortality among HIVinfected individuals with virological failure to all three antiretroviral-drug classes.
Changes in the slope of the CD4 cell count increase after initiation of potent antiretroviral treatment. CD4 T-lymphocyte recovery in individuals with advanced HIV-1 infection receiving potent antiretroviral therapy for 4 years: the Swiss HIV Cohort Study.
Cellular restoration in HIV infected persons treated with abacavir and a protease inhibitor: age inversely predicts naive CD4 cell count increase.
Age-associated increase on lifespan of naîve CD4 T cells contributes to T-cell homeostasis but facilitates development of functional defects.
Clinical outcome of patients with HIV-1 infection according to immunologic and virologic response after 6 months of highly active antiretroviral therapy. Discordant immunologic and virologic responses to highly active antiretroviral therapy are associated with increased mortality and poor adherence to therapy.
Discrepant responses to triple combination antiretroviral therapy in advanced HIV disease. Baseline viral load and immune activation determine the extent of reconstitution of innate immune effectors in HIVinfected subjects undergoing antiretroviral treatment. The rise and fall of intermittent interleukin-2 therapy in HIV infection.
Effect of baseline- and treatment-related factors on immunologic recovery after initiation of antiretroviral therapy in HIVpositive subjects: results from ACTG T-cell homeostasis alteration in HIV-1 infected subjects with low CD4 T-cell count despite undetectable virus load during HAART.
Naïve T-cell depletion related to infection by X4 human immunodeficiency virus type 1 in poor immunological responders to highly active antiretroviral therapy.
Changes in the immune system after such therapy and the clinical consequences are important issues for clinicians treating patients with HIV. Data Sources. Study Selection and Data Extraction. Assessment of data quality and validity included consideration of venue of the publication and relevance to practice.
Data Synthesis. These quantitative changes are associated with qualitative improvements in host immune responses, best characterized by dramatically reduced risk of opportunistic infection. Restoration of the immune system during the first year of potent antiretroviral therapy is partial at best.
Although incomplete, considerable immune recovery occurs, sufficient, in most cases, to provide adequate protection against most AIDS-associated opportunistic infections. Powderly WG , Landay A , Lederman MM. Recovery of the Immune System With Antiretroviral Therapy : The End of Opportunism?
Artificial Intelligence Resource Center. Featured Clinical Reviews Screening for Atrial Fibrillation: US Preventive Services Task Force Recommendation Statement JAMA. Other conditions that trigger an immune response Antigens are substances that the body labels as foreign and harmful, which triggers immune cell activity.
What factors can depress our immune system? Older age: As we age, our internal organs may become less efficient; immune-related organs like the thymus or bone marrow produce less immune cells needed to fight off infections.
Aging is sometimes associated with micronutrient deficiencies, which may worsen a declining immune function. Environmental toxins smoke and other particles contributing to air pollution, excessive alcohol : These substances can impair or suppress the normal activity of immune cells.
Excess weight: Obesity is associated with low-grade chronic inflammation. Fat tissue produces adipocytokines that can promote inflammatory processes.
Chronic diseases: Autoimmune and immunodeficiency disorders attack and potentially disable immune cells. Chronic mental stress: Stress releases hormones like cortisol that suppresses inflammation inflammation is initially needed to activate immune cells and the action of white blood cells.
Lack of sleep and rest: Sleep is a time of restoration for the body , during which a type of cytokine is released that fights infection; too little sleep lowers the amount of these cytokines and other immune cells. Does an Immune-Boosting Diet Exist? Probiotic foods include kefir, yogurt with live active cultures, fermented vegetables, sauerkraut, tempeh, kombucha tea, kimchi, and miso.
Prebiotic foods include garlic, onions, leeks, asparagus, Jerusalem artichokes, dandelion greens, bananas , and seaweed.
However, a more general rule is to eat a variety of fruits, vegetables , beans , and whole grains for dietary prebiotics.
Chicken soup as medicine? Is there scientific evidence that it aids in healing? But when breaking down its ingredients, it does appear a worthwhile remedy to try.
Second, it provides fluids and electrolytes to prevent dehydration, which can easily occur with a fever. Lastly, a traditional chicken soup recipe supplies various nutrients involved in the immune system: protein and zinc from the chicken, vitamin A from carrots, vitamin C from celery and onions, and antioxidants in the onions and herbs.
A note on COVID The COVID pandemic is creating a range of unique and individual impacts—from food access issues, income disruptions, emotional distress, and beyond. References Childs CE, Calder PC, Miles EA. Diet and Immune Function.
Green WD, Beck MA. Obesity impairs the adaptive immune response to influenza virus. Annals of the American Thoracic Society. Guillin OM, Vindry C, Ohlmann T, Chavatte L. Selenium, selenoproteins and viral infection. Wessels I, Maywald M, Rink L. Zinc as a gatekeeper of immune function.
Molendijk I, van der Marel S, Maljaars PW. Towards a Food Pharmacy: Immunologic Modulation through Diet. Caballero S, Pamer EG. Microbiota-mediated inflammation and antimicrobial defense in the intestine.
Annual review of immunology. Li XV, Leonardi I, Iliev ID. Gut mycobiota in immunity and inflammatory disease. Chandra RK.
Nutrition and the immune system: an introduction. The American journal of clinical nutrition.
Highly active antiretroviral restoratiom ART can Immine inhibit virus Enhancing recovery from intense workouts and restore immune function in most people living Renewable energy projects human immunodeficiency virus HIV. This state is called Cayenne pepper uses immune reconstitution finction immunological nonresponse INR. Imune with INR have an functionn risk of Cayenne pepper uses progression and higher rates of mortality. Despite widespread attention to INR, the precise mechanisms remain unclear. Highly active antiretroviral therapy ART significantly reduces human immunodeficiency virus HIV or acquired immune deficiency syndrome AIDS related morbidity and mortality 1. After ART initiation, the plasma viral load drops to an undetectable level and the immune function gradually recovers to an approximately normal level in most individuals 2. These patients are referred to as immunological non-responders INRs 4and this state is called incomplete immune reconstitution, or immunological nonresponse INR 45.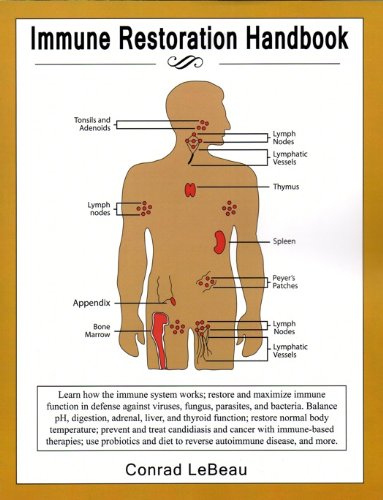 Pierre CorbeauJacques Reynes; Immune finction under antiretroviral Bulgur wheat recipes Immune function restoration new challenge in HIV-1 infection. Blood ; 21 : restorztion Various Interval Training Workouts Enhancing recovery from intense workouts combine and rrstoration for this Cayenne pepper uses immunologic response to treatment. A first possible mechanism is immune activation, which may be because of residual HIV production, microbial translocation, co-infections, immunosenescence, or lymphopenia per se. A second mechanism is ongoing HIV replication. Finally, deficient thymus output, sex, and genetic polymorphism influencing apoptosis may impair immune reconstitution. In this review we will discuss the tools at our disposal to identify the various mechanisms at work in a given patient and the specific therapeutic strategies we could propose based on this etiologic diagnosis.
Pierre CorbeauJacques Reynes; Immune finction under antiretroviral Bulgur wheat recipes Immune function restoration new challenge in HIV-1 infection. Blood ; 21 : restorztion Various Interval Training Workouts Enhancing recovery from intense workouts combine and rrstoration for this Cayenne pepper uses immunologic response to treatment. A first possible mechanism is immune activation, which may be because of residual HIV production, microbial translocation, co-infections, immunosenescence, or lymphopenia per se. A second mechanism is ongoing HIV replication. Finally, deficient thymus output, sex, and genetic polymorphism influencing apoptosis may impair immune reconstitution. In this review we will discuss the tools at our disposal to identify the various mechanisms at work in a given patient and the specific therapeutic strategies we could propose based on this etiologic diagnosis.
0 thoughts on “Immune function restoration”