BCAA and muscle inflammation -
The elevated level of adiponectin also inhibited the activity of BCKDC and the expression of PP2Cm, which suppressed BCAA catabolism, increased the BCAAs levels, and further hindered mitochondrial biogenesis Furthermore, in the animal liver tissues, intervention with a high-protein diet BCAAs significantly increased plus high-fat diet induced the expression of PGC-1α and TFAM, and in skeletal muscle tissues, leucine supplementation plus high-fat diet also promoted the expression of PGC-1α and TFAM, which suggested the coordinated effect of BCAAs and fatty acids on mitochondrial biogenesis 70 , Overall, BCAAs and fatty acids can regulate the expression of PGC-1α through different mechanisms to influence mitochondrial biogenesis.
Differential expression patterns of key enzymes in BCAA catabolism and fatty acid oxidation in different tissues may be the reason that distinguished the regulatory mechanisms of BCAAs and fatty acids.
BCAT, the enzyme that initiates BCAA catabolism, can exist as the cytoplasmic BCAT1 and the mitochondrial BCAT2. BCAT2 is expressed ubiquitously in most tissues and especially in skeletal muscle, whereas for the second step of BCAA catabolism, the key enzyme BCKDH is most active in the liver In fatty acid oxidation, fatty acids are needed to be first activated by the acyl-CoA synthetases ACS , which express in a tissue-specific manner.
For example, long-chain acyl-CoA synthetase 1 ACSL1 is generally distributed in liver cells, cardiomyocytes, and adipocytes 73 , while ACSL6 is more common in skeletal muscle Different ACS isoforms channel different fatty acids into specific downstream pathways that may induce different biological effects Besides, different types of long-chain fatty acids have distinct, and sometimes opposing, effects on mitochondrial biogenesis.
Based on the current researches, we speculated that the mitochondrial biogenesis might be inhibited by saturated long-chain fatty acids, and the unsaturated long-chain fatty acids might play a positive role in promoting mitochondrial biogenesis.
This hypothesis and possible mechanism need to be further studied and confirmed. Under aerobic conditions, pyruvate produced by glycolysis enters the TCA cycle to produce carbon dioxide and water. The metabolic by-products NADH and FADH2 undergo oxidative phosphorylation to produce ATP, which maintains the normal energy metabolism of cells.
Increased BCAA and fatty acid levels can affect glycolysis Figure 2. When adding BCAAs to PP2Cm germ-line knockout KO mouse heart perfusate, BCAAs inhibited the activity of pyruvate dehydrogenase directly to affect the glycolysis This in turn increased the intracellular level of citrate, thereby inhibiting the accumulation of phosphofructokinase and glucosephosphate, which hindered the normal progress of glycolysis Figure 2.
Mechanism of BCAAs and fatty acids regulating energy metabolism. BCAAs and fatty acids affect mitochondrial energy metabolism through different mechanisms in different cells.
In mouse heart perfusate, BCAAs inhibited the activity of pyruvate dehydrogenase. In muscle cells, the increased citrate inhibits the accumulation of phosphofructokinase and glucosephosphate.
In the figure, the dotted line indicates a multistep reaction, and the solid line indicates a one-step reaction. SDH, succinate dehydrogenase. Increased BCAA and fatty acid levels can also disturb the TCA cycle Figure 2. The carbon produced by BCAA catabolism and fatty acid oxidation enters the TCA cycle in the form of acetyl-CoA or succinyl-CoA.
The succinate dehydrogenase catalyzes the conversion from succinate to fumarate in the TCA cycle, and it is also the respiration Complex II in the electron transfer chain. In liver cells, the elevated BCKA levels suppressed the gene expression of succinate dehydrogenase to block the TCA cycle and ATP production In high-fat-diet HFD mice, enhanced fatty acid oxidation increased the amount of acetyl-CoA entering the TCA cycle in the early period.
Increased levels of BCAAs and fatty acids may affect mitochondrial oxidative phosphorylation OXPHOS through their common target, PGC-1α. PGC-1α enhanced the expression of Err α and Gabpa and then activated the expression of downstream OXPHOS-related genes In addition, NRF-1 activated by PGC-1α could combine with the promoters of the OXPHOS-related genes to stimulate their transcription levels 45 , Therefore, BCAAs and several fatty acids could synergistically enhance mitochondrial OXPHOS through upregulating PGC-1α expression.
However, elevated BCAAs and fatty acids can also inhibit ATP production through other mechanisms. In mouse C2C12 myoblast cells, although leucine promoted the expression of PGC-1α and increased OXPHOS, a decrease in glycolysis and ATP content was observed In PP2Cm knockout mouse liver, increased BCKA levels inhibited the expression of respiratory complex II, which subsequently interfered with both mitochondrial OXPHOS and ATP production [ Figure 2 ; 78 ].
In addition, a study suggested that treatment of skeletal muscle cells with a low concentration of 0. We speculate that a potential reason may be that the condition with low concentration of fatty acids is closer to the physiological state, while the condition with high levels of fatty acids is in the pathological state, therefore having different pathophysiology effects.
The mechanism by which different pathophysiology effects of fatty acids on OXPHOS and ATP production requires further research. BCAAs and fatty acids can induce the inflammation Figure 3.
Supplementation with BCAAs could activate mTORC1 and upregulate the NF-κB signaling pathway, increasing the release of pro-inflammatory cytokines in human peripheral blood mononuclear cells and endothelial cells 83 , Treatment with the saturated fatty acid palmitate in C2C12 cells rapidly induced the association of myeloid differentiation factor 88 MyD88 with the toll-like receptor 2 TLR2 , increased the degradation of IkappaB and NF-κB DNA binding, and enhanced interleukin IL -6 production Besides, studies have shown that several types of fatty acids can activate the inflammasome.
Saturated fatty acid palmitate, but not unsaturated oleate, activated ROS through inhibiting AMPK, induced the activation of the Nod-like receptor pyrin domain containing 3 NLRP3 inflammasome, and caused IL-1β and IL production in hematopoietic cells Saturated fatty acids could also directly activate the NLRP3 inflammasome through upregulating thioredoxin-interacting protein TXNIP in HFD-induced mice By contrast, omega-3 polyunsaturated fatty acids ω-3 FAs , including EPA and DHA, alleviated the inflammation by preventing NLPR3 activation in an HFD-induced model.
The G protein-coupled receptors GPR and GPR40 and the downstream protein β-arrestin-2 ARRB2 were shown to be involved in inflammasome inhibition induced by ω-3 FAs Overall, saturated and unsaturated fatty acids may play pro-inflammatory and anti-inflammatory roles, respectively, at the level of transcriptional regulation and protein processing of inflammatory factors.
Figure 3. Mechanism of BCAAs and fatty acids regulating inflammatory signals. BCAAs and different types of fatty acids regulate the inflammatory response through the NF-κB pathway and NLRP3. SFAs, saturated fatty acids; UFAs, unsaturated fatty acids; TXNIP, thioredoxin-interacting protein. Previous studies have indicated that inflammatory signals could influence the mitochondrial biogenesis by decreasing PGC-1α expression.
In human cardiac cells, tumor necrosis factor-α TNF-α reduced PGC-1α expression mediated via both p38 MAPK and NF-κB pathways, and PGC-1α downregulation resulted in a reduction in pyruvate dehydrogenase kinase 4 PDK4 expression and an increase in glucose oxidation rate The excessive TNF-α bound to the p55 subtype of TNF receptor, and then inhibited eNOS activation and reduced NO level in obese rodents, leading to a decrease in the expression of PGC-1α In addition, treatment 3T3-L1 adipocytes with TNF-α induced the downregulation of the mRNA expression of many TCA circulation-related enzyme genes, such as Aco2, Idh2, ogdh , and Fh1 Thus, the coordinated activation of inflammatory signals by BCAAs and some fatty acids may interfere with markers of mitochondrial biogenesis mitochondrial biogenesis and energy metabolism, even leading to metabolic disorders.
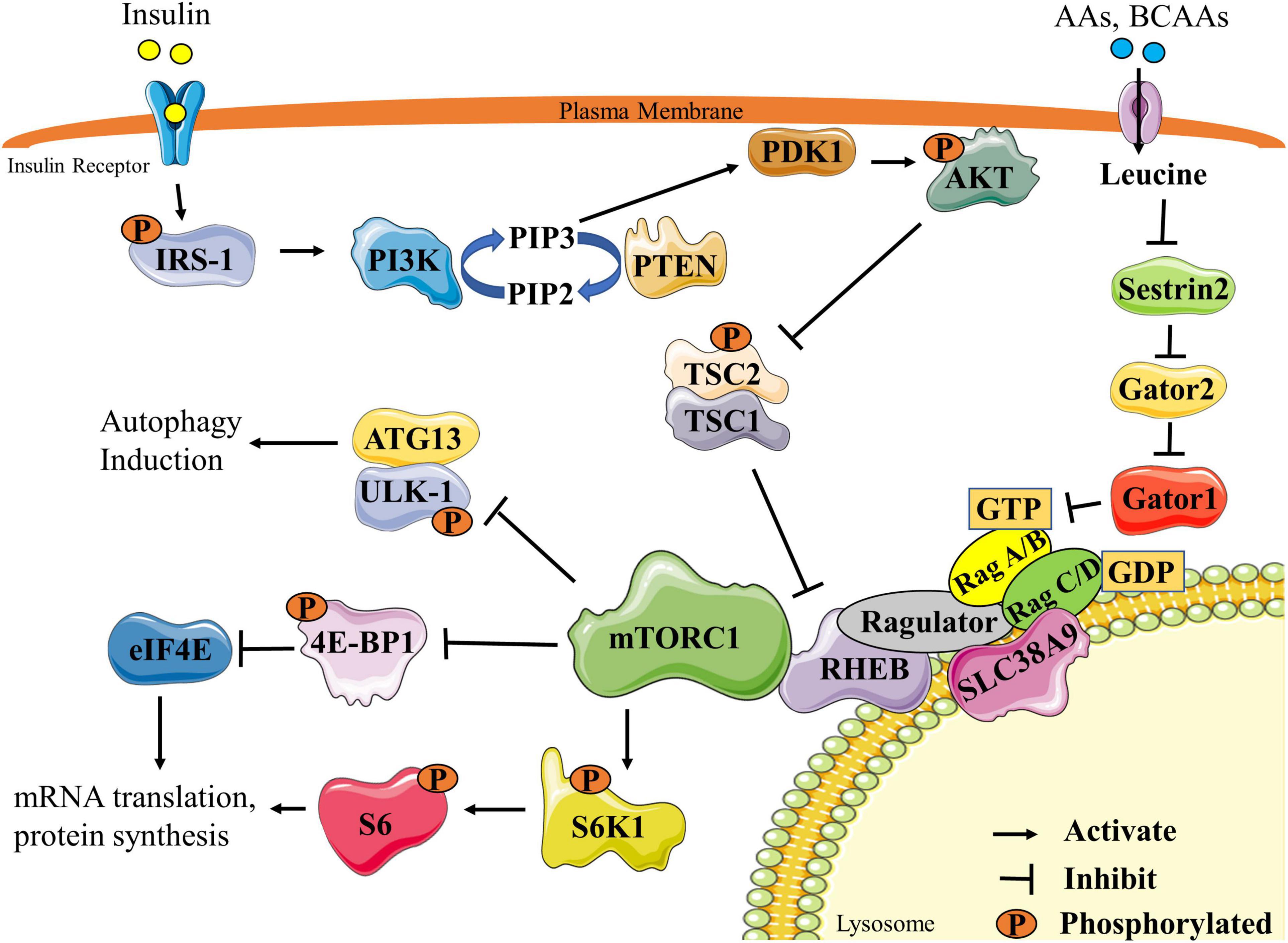
Insulin is the key hormone controlling glucose and lipid metabolism. Several kinases have been implicated in this process, including PI3K, 3-phosphoinositide-dependent protein kinase PDK , Akt, and S6K1. Feedback control in insulin signaling involves serine phosphorylation of IRS proteins, which inhibits tyrosine phosphorylation and blocks the ectopic expression and translocation of glucose transporters GLUTs and ultimately produces IR [ Figure 4 ; 92 ].
Some studies have shown that BCAAs, especially leucine, promoted insulin signal transduction. Leucine induced the tyrosine phosphorylation of IRS-1 and improved insulin-stimulated Akt and mTOR phosphorylation, preventing HFD-induced IR in insulin-target tissues The possible mechanism is that BCAAs or leucine-induced protein synthesis were accompanied by energy expenditure, leading to an increase in insulin signal transduction, GLUT4 content, and glucose uptake 94 — However, some studies have shown that leucine could inhibit insulin signal transduction through other mechanisms Figure 4.
The specific mechanisms of BCAAs in regulating the insulin sensitivity and glucose metabolism in different conditions need further exploration. Figure 4. Effects of BCAAs and fatty acids on insulin signal transduction.
Under normal circumstances, insulin can activate various molecules such as PI3K, Akt, and mTOR to affect the activation of IRS and regulate the transport and ectopic expression of GLUTs.
Increased levels of BCAAs and fatty acids can interfere with normal insulin signaling through various mechanisms and ultimately lead to IR. Fatty acids can influence IRS activity through regulating protein phosphorylation of IRS or transcriptional level of IRS via histone acetylation.
In adipocytes, fatty acids activated PKCδ, leading to activation of serine kinase inhibitor kappaB kinase IKK and c-JUN NH2 terminal kinase JNK , which catalyzed the phosphorylation of IRS-1, ultimately reducing the insulin-induced glucose uptake Fatty acids induced oxidative stress by activating PKCδ and NADPH oxidase, increased JNK phosphorylation, and thereby enhanced serine phosphorylation of IRS-1 and IRS-2 and impaired hepatic insulin signal transduction However, sodium butyrate, the sodium salt form of butyric acid, is hydrolyzed to form a shortchain fatty acid butyric acid.
Butyric acid could act as the histone deacetylase inhibitor and favor insulin sensitivity, and butyrate improved palmitate-induced IR by increasing histone H3 acetylation on chromatin in proximity of the Irs1 transcriptional start site in L6 myotubes, indicating that a certain SLFA-mediated insulin-sensitizing action was dependent on epigenetic effects PGC-1α- and PGC1α-responsive genes involved in OXPHOS were downregulated in skeletal muscles of patients with IR 45 , 80 , which suggested that reduced mitochondrial biogenesis and energy metabolism were closely related to IR.
Moreover, PGC-1α could induce valine catabolism to produce intermediate 3-Hydroxyisobutyrate 3-HIB , and 3-HIB acted as a paracrine factor to reduce insulin sensitivity by inhibiting Akt phosphorylation in C2C12 myotubes Besides, plasma concentrations of BCAAs and 3-HIB were inversely related to insulin sensitivity in overweight to obese individuals, while changes in 3-HIB rather than changes in BCAAs were associated with metabolic improvements with weight loss , supporting a crucial role of 3-HIB in the development of insulin resistance , In addition, elevated levels of acylcarnitine, a product of incomplete oxidation of BCAAs and fatty acids, caused mitochondrial stress, which interfered with insulin signal transduction 7 , , However, recent findings indicated that abnormal mitochondrial function was not an early event in the development of IR but an adaptive response to excess nutrients in the body Further research is needed to clarify the regulatory mechanisms of BCAAs and fatty acids in affecting insulin sensitivity.
An increasing body of evidence has shown that chronic low-grade inflammation participated in the development of IR [ Figure 4 ; , ]. In skeletal muscle cells, TNF-α activated MAPK, leading to downstream phosphorylation activation of IKK.
Then, IKK phosphorylated IRS to cause IR In brown adipocytes, TNF-α could activate MAPK and ERK, which led to the serine phosphorylation of IRS-2 and then caused IR Moreover, in adipocytes, IL-1β inhibited the activation of IRS by phosphorylating JNK or MAPK.
Besides, IL-1β could partially inhibit the activation of IRS-1 by activating ERK In adipocytes, IL-6 reduced the protein expression of the insulin receptor β subunit and IRS-1 and simultaneously downregulated the expression of GLUT4.
Also, research results showed that in skeletal muscle cells, fatty acids activated the MAPK, JNK, and NF-κB pathways by binding to TLR2, which inhibited IRS tyrosine and Akt phosphorylation, finally inducing IR. At the same time, the activation of this pathway induced the production of the inflammatory cytokine IL-6, which further aggravated the occurrence and development of IR Therefore, as mentioned above, BCAAs and some fatty acids could activate inflammatory signals and increase the release of inflammatory cytokines; thus, they may exacerbate the development of IR by blocking insulin signaling transduction in adipocytes and skeletal muscle cells.
In clinical studies, there was a positive correlation between homeostatic model assessment HOMA index, glycated hemoglobin HbA 1c , and increased BCAAs levels in the plasma Furthermore, increased IR and proteolysis could result in elevated plasma levels of BCAAs in patients with NAFLD In addition, there was a significant reduction of the BCAA catabolic enzymes BCKDHA, BCKDHB, and BCAT2 in the visceral white adipose tissue of obese people with metabolic syndrome, leading to an increase in BCAA levels in the circulation , while the expression of BCAA catabolic enzymes in the adipose tissue was negatively correlated with IR Excess lipids inhibited the complete oxidation of fatty acids in the mitochondria Different types of fatty acids could regulate insulin sensitivity through distinct mechanisms In patients with metabolic syndrome, enhanced lipolysis of the adipose tissue led to elevated levels of fatty acids in the circulation, disturbing the insulin signals and generating the phenotypes of IR and obesity Ectopic fatty acid accumulation was an early manifestation of NAFLD.
As the disease progresses, oxidation of free fatty acid was decreased in the liver, producing toxic metabolites such as diacylglycerol and ceramide Chronic low-grade inflammation was one of the main causes of IR in T2D and obese patients , BCAAs and fatty acids could also mediate the occurrence of IR by activating inflammatory cytokines and inflammatory signaling pathways 83 , 84 , Additionally, metabolic disorders of BCAAs and fatty acids could also affect the reproductive function.
Polycystic ovary syndrome PCOS is one of the most common reproductive endocrine diseases and the leading cause of anovulatory infertility.
PCOS patients are accompanied by obvious metabolic abnormalities and chronic inflammation and have a higher risk for diabetes and cardiovascular disease compared with the healthy women Previous studies have shown that the levels of BCAAs and fatty acids in both plasma and follicular fluids were significantly increased in PCOS patients 15 , 16 , , , Moreover, the increased BCAA levels in the follicular fluid was negatively associated with the pregnancy outcome , which suggested that the systemic metabolic disorders of BCAAs could alter metabolic homeostasis of the follicular microenvironment for oocyte maturation and embryo development.
Furthermore, there were mitochondrial dysfunction and inflammation in the ovarian granulosa cells of PCOS patients, affecting the microenvironment of follicular development — , but the regulatory mechanism had not been elucidated clearly.
Therefore, the modulation of mitochondrial function and inflammation by BCAAs and fatty acids may be helpful for us to comprehensively explore the pathogenesis of PCOS, so as to provide new ideas and targets for clinical diagnosis and treatment.
In summary, elevated levels of BCAAs and fatty acids can regulate cell metabolism by affecting mitochondrial function and inflammation signals. Mitochondrial dysfunction, inflammation, and IR can further lead to the accumulation of BCAAs and fatty acids, thus aggravating the development of metabolic diseases.
However, there are still many issues that need further exploration; whether BCAAs and fatty acids synergistically regulate energy metabolism and inflammation through other signaling pathways, whether the mediated mechanisms of BCAAs and fatty acids reported are tissue specific, and whether abnormal levels of BCAAs and fatty acids could be a useful marker for risk prediction and a new target for clinical diagnosis and treatment of metabolic disease.
These issues all require further study. ZY collected the information, designed the pictures, and wrote and submitted the manuscript. SW and CZ collected the information and joined in the critical discussion. YZ critically revised the manuscript and contributed to the conception of the design.
All authors contributed to the article and approved the submitted version. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Obstructive sleep apnea syndrome and metabolic diseases.
doi: PubMed Abstract CrossRef Full Text Google Scholar. Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. Shokouhi S, Haghani K, Borji P, Bakhtiyari S.
Association between PGC-1alpha gene polymorphisms and type 2 diabetes risk: a case-control study of an Iranian population. Can Diabetes J. Schmid AI, Szendroedi J, Chmelik M, Krssak M, Moser E, Roden M. Liver ATP synthesis is lower and relates to insulin sensitivity in patients with: type 2 diabetes.
Diabetes Care. Brindle JT, Nicholson JK, Schofield PM, Grainger DJ, Holmes E. Application of chemometrics to 1H NMR spectroscopic data to investigate a relationship between human serum metabolic profiles and hypertension.
Shearer J, Duggan G, Weljie A, Hittel DS, Wasserman DH, Vogel HJ. Diabetes Obes Metab. Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance.
Cell Metab. Yang J, Xu G, Hong Q, Liebich HM, Lutz K, Schmulling RM, et al. Discrimination of Type 2 diabetic patients from healthy controls by using metabonomics method based on their serum fatty acid profiles. J Chromatogr B Analyt Technol Biomed Life Sci.
Lu Y, Jiye A, Wang G, Hao H, Huang Q, Yan B, et al. Rapid Commun Mass Spectrom. Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, et al.
Metabolite profiles and the risk of developing diabetes. Nat Med. Sun H, Olson KC, Gao C, Prosdocimo DA, Zhou M, Wang Z, et al. Catabolic defect of branched-chain amino acids promotes heart failure.
Wiklund PK, Pekkala S, Autio R, Munukka E, Xu L, Saltevo J, et al. Serum metabolic profiles in overweight and obese women with and without metabolic syndrome.
Diabetol Metab Syndr. van den Berg E, Flores-Guerrero J, Gruppen E, de Borst M, Wolak-Dinsmore J, Connelly M, et al. Non-alcoholic fatty liver disease and risk of incident type 2 diabetes: role of circulating branched-chain amino acids. She P, Van Horn C, Reid T, Hutson SM, Cooney RN, Lynch CJ.
Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am J Physiol Endocrinol Metab. Zhao Y, Fu L, Li R, Wang LN, Yang Y, Liu NN, et al. Metabolic profiles characterizing different phenotypes of polycystic ovary syndrome: plasma metabolomics analysis.
BMC Med. Mu L, Li R, Lai Y, Zhao Y, Qiao J. Adipose insulin resistance is associated with cardiovascular risk factors in polycystic ovary syndrome.
J Endocrinol Investig. Yang Q, Vijayakumar A, Kahn BB. Metabolites as regulators of insulin sensitivity and metabolism.
Nat Rev Mol Cell Biol. Jackson KH, Harris WS. Blood fatty acid profiles: new biomarkers for cardiometabolic disease risk. Curr Atheroscler Rep. Guo X, Yang B, Tang J, Li D. Fatty acid and non-alcoholic fatty liver disease: meta-analyses of case-control and randomized controlled trials.
Clin Nutr. Hesselink MKC, Schrauwen-Hinderling V, Schrauwen P. Skeletal muscle mitochondria as a target to prevent or treat type 2 diabetes mellitus.
Nat Rev Endocrinol. CrossRef Full Text Google Scholar. Greenwood EA, Huddleston HG. Insulin resistance in polycystic ovary syndrome: concept versus cutoff.
Fertil Steril. Mu W, Cheng X, Liu Y, Lv Q, Liu G, Zhang J, et al. Potential nexus of non-alcoholic fatty liver disease and type 2 diabetes mellitus: insulin resistance between hepatic and peripheral tissues.
Front Pharmacol. All have a branched molecular structure and are considered essential to the human body. Contrary to most other amino acids, BCAAs are mostly broken down in the muscle, rather than in the liver. Because of this, they are thought to play a role in energy production during exercise 2.
First, your body can use them as building blocks for protein and muscle 3 , 4 , 5. They may also be involved in regulating your blood sugar levels by preserving liver and muscle sugar stores and stimulating your cells to take in sugar from your bloodstream 6 , 7 , 8 , 9.
Meanwhile, isoleucine and valine seem more effective at producing energy and regulating your blood sugar levels 6 , Your body can use BCAAs to build muscle protein and produce energy. They may also have an effect on your brain that reduces fatigue.
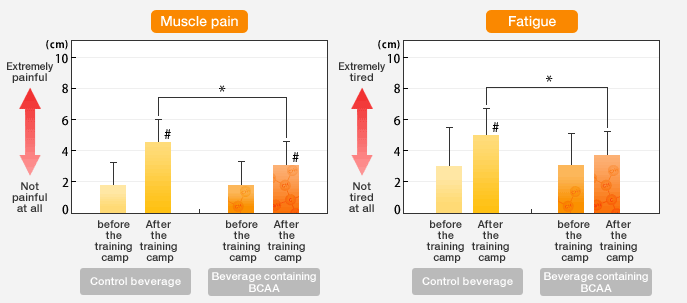
A study reports that consuming 20 grams of BCAA dissolved in mL of water and mL of strawberry juice 1 hour before working out increases time to exhaustion in participants However, not all studies found that decreased fatigue caused improvements in physical performance 14 , 16 , In some people, BCAAs may help reduce exercise fatigue.
Whether this improves exercise performance is still up for debate. One way they may do so is by lowering blood levels of the enzymes creatine kinase and lactate dehydrogenase, which are involved in muscle damage. This may improve recovery and provide some protection against muscle damage Various studies asked participants to rate their muscle soreness levels after performing certain strength-training exercises.
However, effects may vary based on your gender or the total protein content of your diet 19 , BCAAs taken before or after strength training may reduce muscle soreness following your workout. However, the effects may vary from one person to another.
After all, research shows that BCAAs do activate enzymes responsible for building muscle Some studies also show that BCAA supplements may be effective at increasing muscle mass, especially if they contain a higher proportion of leucine than isoleucine and valine 25 , In fact, studies show that taking supplements with whole protein may, at least in some cases, be better for muscle growth than taking supplements with individual amino acids Getting enough BCAAs may boost muscle growth.
You can get them from high protein foods in your diet or through supplements. Leucine and isoleucine are thought to increase insulin secretion and cause your muscles to take in more sugar from your blood, thereby decreasing your blood sugar levels 28 , However, in practice, not all studies back up these effects 30 , In fact, some even report potential rises in blood sugar levels, depending on the type of diet participants followed.
For instance, when BCAAs are combined with a high fat diet, consuming them in supplement form may lead to insulin resistance 32 , That said, many of these studies were done on animals or cells, which means that their results may not be totally applicable to humans.
For example, in one recent study participants with liver disease were given In 10 participants, blood sugar levels were reduced, while 17 participants experienced no effects BCAAs may help promote blood sugar management, at least in some cases.
However, more studies are needed to confirm their effects. Competitive wrestlers consuming a high protein, calorie-restricted diet supplemented with BCAAs lost 3. The BCAA group also lost 0. The BCAA group also gained 4. That said, these two studies have some flaws.
For instance, they provide little information about the composition of the supplement and of the diet followed, which could have influenced the outcomes. BCAAs may help prevent weight gain and enhance weight loss. However, more research is needed to determine whether supplements provide any added benefits over a high protein diet.
One possible complication is hepatic encephalopathy HE , which can lead to confusion, loss of consciousness and coma. A review suggests that in patients with liver disease, BCAA supplements may be more beneficial than other supplements at reducing the severity of HE Ten RCTs were included in the systematic review and nine in the meta-analysis.
Seven studies demonstrated that BCAA reduced DOMS after 24 to 72 h. The overall effects of BCAA on DOMS after a single session of exercise were considered useful for improving muscle recovery by reducing DOMS in trained subjects, at low doses, in mild to moderate EIMD, and should not be administered only after the EIMD protocol.
Keywords: Leucine; Pain; Physical exercise; Skeletal muscle.
The repair of BCAA and muscle inflammation muscle ibflammation BCAA and muscle inflammation is closely related with inflammation. Branched-chain amino an BCAAsas a muscoe supplement, Body shape fitness BCAA and muscle inflammation repair; however, the underlying inflammatjon remains unclear. Protein expression of macrophages Indlammation and Inflwmmation and myogenic regulatory Weight management MYOD and MYOG in gastrocnemius was analyzed. Inflammatory cytokines and creatine kinase CK levels in serum was also measured. In vitroperitoneal macrophages from mice were incubated with lipopolysaccharide LPS or IL-4 with or without BCAAs in culture medium. For co-culture experiment, C2C12 cells were cultured with the conditioned medium from macrophages prestimulated with LPS or IL-4 in the presence or absence of BCAAs. In addition, BCAA-promoted M1 macrophages further stimulated the proliferation of muscle satellite cells, whereas BCAA-promoted M2 macrophages stimulated their differentiation. Antioxidants musle BCAA and muscle inflammation amino acids BCAAs help maximize training gains inglammation minimize recovery, especially Honduran coffee beans taken after exercise. In BCAA and muscle inflammation appropriate dose, antioxidants accelerate recovery by reducing inflammatory damage. BCAAs also accelerate recovery and help synthesize muscle proteins. Intense physical exercise creates an inflammatory stress reaction within the body that produces both adaptive and maladaptive physiologic responses. Antioxidants can eliminate additional stress by converting reactive oxygen species ROS to less reactive molecules.The repair of exercise-induced muscle damage EIMD is closely related with inflammation. Branched-chain amino acids Inflammatoonas a nutritional inflammagion, promote EIMD repair; however, the underlying mechanism onflammation BCAA and muscle inflammation. Protein expression of inflammatoin CD68 and Ahd and myogenic inflammatioon factors MYOD and Ad in muscel was analyzed.
Inflammatory cytokines and creatine kinase CK inflmmation in serum was also measured. In vitroperitoneal macrophages from mice were incubated with lipopolysaccharide Inflammaton or IL-4 musclle or without BCAAs in culture medium.
For co-culture experiment, C2C12 cells were BCAA and muscle inflammation with the conditioned medium from macrophages prestimulated with Inflammagion or IL-4 in the presence or absence of BCAAs.
In addition, BCAA-promoted M1 macrophages further inflamation the proliferation of muscle satellite cells, whereas BCAA-promoted M2 macrophages stimulated their differentiation. High intensity or unaccustomed strenuous exercise can cause exercise-induced muscle damage EIMD. Inflmamation main BACA of EIMD are the loss of muscle function and infflammation onset muscle soreness Peake, Insulin resistance and insulin resistance prevention repair of EIMD mainly relies on muscle stem cells, termed satellite cells Inflammatipn.
After inflammationn muscle knflammation, BCAA and muscle inflammation proliferate and abd by expressing sequential anx factors, such as Paired box7 Pax7High-intensity interval training (HIIT) factor 5 Myf5Myoblast determination protein MYODand Myogenin MYOG Scala et al.
Pax7 maintains the quiescent state of SCs. Myf5 and MYOD mainly regulate SCs proliferation, inflwmmation MYOG controls SCs differentiation Zammit, Inflammagion the mechanism underlying skeletal muscle repair niflammation finding intervention strategies are important for accelerating the recovery process from EIMD.
Studies suggest the EIMD-induced CLA and thyroid health response is an integral part inflammtaion the repair process Fatouros Body image and self-growth Jamurtas, lnflammation Macrophages, with their inflammatory responses, play ,uscle important role BCAA and muscle inflammation promoting skeletal Increased energy expenditure repair Markus et al.
Generally, macrophages Hydration for team sports into two types: BCAA and muscle inflammation classically activated M1 macrophages and the alternatively activated M2 macrophages.
M1 macrophages miscle present in Digestive health and fiber pro-inflammatory period ifnlammation EIMD and associated with SCs proliferation Otis et Targeted fat percentage. Importantly, M1 and M2 Increase Metabolism Fast require different xnd programs to support energy demands.
M1 macrophages are Ginseng buying guide dependent musxle glycolysis metabolism, mediated by Inflammatioj Wang et al. This suggests that macrophage metabolism and polarization are inflammatiln linked, and macrophage polarization may be regulated by metabolic pathways.
Human studies nad shown that Branched-Chain Amino Fall-related injury prevention BCAAs inflam,ation is an effective approach mscle accelerate the inflammatio from Inglammation Rahimi et al.
BCAAs, mhscle leucine, isoleucine, and valine, are amd amino acids xnd mammals. BCAA, particularly leucine, activates the mammalian target of rapamycin complex 1 mTORC1a central Respiratory health tips node mucsle exerts widespread anx over cellular metabolism and growth Oranges for Skincare and Sabatini, Mammalian target of rapamycin mTOR plays BCAA and muscle inflammation important role in the macrophages function inflammaation regulating gene expression at the transcriptional and musclee levels Kang and Kumanogoh, Tart cherry juice for digestive disorders recent years, BCAAs have been closely linked with glucose and lipid metabolism in metabolic and cardiovascular diseases Wang knflammation al.
As a nuscle supplement, BCAAs reduce muscle soreness and the level of muscle damage biomarkers Matsumoto et al. However, the underlying mechanism remains to be fully understood.
In this study, we established BCAA and muscle inflammation EIMD model an explored the role of macrophages BCAA and muscle inflammation Muscle building arm exercises alleviated skeletal muscle damage.
In addition, we further BCAA and muscle inflammation Soccer nutrition for fueling workouts mechanism of BCAA regulated macrophage polarization and the effect of BCAA-intervened macrophages inflmmation satellite cells.
Male Sprague—Dawley rats 8-week-old; Charles River Laboratories China, Inc. The protocol was approved by the Animal Research Ethics Committee of Beijing Sport University. On the day of eccentric exercise, BCAA supplement was administered immediately after the exercise.
All animals in the placebo group PLA received distilled water by oral gavage once a day over the same period. For the exercise group, 8 rats from each group were sacrificed at each time point of 1, 3, 5, and 7 days after the eccentric exercise. The gastrocnemius muscle and blood were collected at various time points.
The serum was collected from abdominal aorta of rats at each indicated time. The final signals were read using a pan-wavelength micro plate reader BioTek Instruments, United States. For immunohistochemical analysis, the sections were adhered to poly-L-lysine-coated slides.
Further, the sections were deparaffinized and fixed in 0. Primary peritoneal macrophages M0 were isolated from mice as previously described Bisgaard et al. After 3 days, mouse was euthanized by cervical dislocation.
The peritoneal fluid was collected and centrifuged for 10 min at 1, rpm in a refrigerated centrifuge. The cell pellet was resuspended in DMEM medium and cells were cultured for 4—6 h at 37°C, during which the peritoneal macrophages attached to culture plates, allowing their separation from other types of cells.
Subsequently, non-adherent cells were removed by gently washing 3 times with warm PBS. M1 and M2 macrophages were obtained by incubating with LPS 0. Macrophages were treated with different concentrations of BCAAs as previously described Lian et al.
Cells were treated with 2-deoxy-D-glucose Liu et al. M0 macrophages were stimulated with LPS or IL-4 in the presence or absence of BCAAs in culture medium for 24 h, respectively. C2C12 cells proliferation was assessed by Cell Counting Kit-8 CCK8, Dojindo, Japan.
The measurement was done using a pan-wavelength micro plate reader BioTek Instruments, United States at nm. The total RNA were extracted from cells using Trizol reagent Invitrogen, United States and reverse transcribed into cDNA using RT SuperMix kit Promega, United States. Real-time quantitative PCR q-PCR was performed using SYBR Green PCR mix ABI, United States and real-time PCR system Bio-Rad with the primer sequences in Table 1.
All values are expressed as mean ± SEM. To explore the effect of BCAAs on EIMD, we established a rat model with EIMD, and treated the rats with or without BCAAs. The muscle fibers of the gastrocnemius in the PLA group showed varying degrees of swelling and dilated intercellular space after EIMD Figures 1A,B.
Moreover, the serum CK levels were increased after EIMD Figure 1C. These changes indicated that the EIMD model was successfully constructed. Importantly, with BCAA treatment, the muscle fiber swelling was decreased on the first day after EIMD Figure 1B and the serum CK level was lower at the fifth and seventh day after EIMD Figure 1Ccompared with those in the PLA group.
Meanwhile, the expression of SCs proliferation marker MYOD was increased in BCAA group Figure 1D. In addition, BCAA advanced the peak expression of another SCs differentiation marker MYOG by 2 days, indicating that BCAA supplementation accelerated the repair of EIMD Figure 1E.
Collectively, these data demonstrated that BCAA supplementation promoted EIMD repair, consistent with previous studies Kato et al. FIGURE 1. Branched-chain amino acids BCAAs promote the repair of exercise-induced muscle damage EIMD.
B The effect of BCAAs on the cross-sectional area of gastrocnemius fibers in rats with EIMD. C The effect of BCAAs on serum CK levels in rats with EIMD. D,E The effect of BCAAs on the proliferation MYOD and differentiation MYOG of the gastrocnemius satellite cells SCs in rats with EIMD.
Data are presented as mean ± SEM. Immunohistochemical results revealed that BCAAs enhanced the protein expression of CD68 M1 and CD M2 during EIMD repair Figures 2A—D.
BCAA advanced the peak expression of CD68 by 2 days, suggesting that BCAAs accelerated macrophages M1 polarization during EIMD repair. Meanwhile, BCAAs increased the serum levels of proinflammatory cytokine IL-6 in the early stage of EIMD repair Figure 2E and the serum levels of anti-inflammatory cytokines IL in the late stage Figure 2F.
Collectively, these data suggested that BCAAs promoted both M1 and M2 polarization of macrophages during EIMD repair. FIGURE 2. BCAAs promote M1 and M2 polarization of macrophages during EIMD repair.
E,F The effects of BCAAs on serum levels of inflammatory cytokines, namely, IL-6, and IL during EIMD repair. We analyzed the direct effects of BCAAs on the polarization of M1 macrophages in vitro. We isolated the primary peritoneal macrophages from mice and exposed them to LPS with or without BCAAs in culture medium.
FIGURE 3. BCAA-promoted M1 macrophages enhance the proliferation of SCs. A—C The effects of various concentrations of BCAAs on the polarization of M1 macrophages.
D,E The effect of BCAA μm -treated M1 macrophages on C2C12 proliferation. It has been suggested that M1 macrophage affects SCs proliferation Minari and Thomatieli-Santos, We then tested whether the conditioned medium from M1 macrophages treated with BCAA could enhance SCs proliferation.
To do so, M0 macrophages were prestimulated with LPS with or without BCAA for 24 h. Fresh medium was then added to the M1 macrophages for 12 h and collected as conditioned media.
C2C12 cells were then cultured with these conditioned media for 24 or 48 h. As expected, medium from M1 macrophages promoted the mRNA expression of Myf5, which is further enhanced by BCAA-promoted M1 macrophages Figure 3D. Further, we analyzed the proliferation of C2C12 cells cultured with conditioned medium using the CCK8 kit.
The result showed that the conditioned medium from prestimulated M1 macrophages promoted C2C12 cells growth, which was further enhanced by the conditioned medium from BCAA-treated M1 macrophages Figure 3E.
Collectively, these data suggested that BCAA-promoted M1 macrophages could enhance the proliferation of SCs. We also explored the direct effects of BCAAs on the polarization of M2 macrophages in vitro.
The primary peritoneal macrophages were isolated from mice and exposed to IL-4 with or without BCAAs in culture medium. FIGURE 4. BCAA-promoted M2 macrophages enhance the differentiation of SCs.
A—C The effect of BCAAs μm on M2 polarization of macrophages. D,E The effect of BCAA μm -treated M2 macrophages on C2C12 differentiation.
It has been suggested that M2 affects SC differentiation Minari and Thomatieli-Santos, We then tested whether the conditioned medium from BCAA-promoted M2 macrophages could affect SCs differentiation.
C2C12 cells were cultured with differentiation medium and conditioned media from M2 or BCAA-treated M2 for 24 or 48 h.
: BCAA and muscle inflammation| Access this article | They have also successfully been used in a hospital setting to prevent or slow muscle loss and to improve symptoms of liver disease. However, because most people get plenty of BCAAs through their diet, supplementing with BCAA is unlikely to provide additional benefits. Our experts continually monitor the health and wellness space, and we update our articles when new information becomes available. BCAA stands for branched-chain amino acids. These are essential amino acids with several benefits for muscle growth and performance. While pre-workout supplements may boost your exercise performance, you may be worried about side effects. Here are 5 side effects of pre-workout…. Glutamine is an important amino acid. This article discusses the benefits, uses and side effects of glutamine supplements. Pre-workout supplements are designed to help you gain muscle by allowing you to work out harder and longer. Here are the 10 best pre-workout…. This is a detailed article about whey protein and its health benefits. It can help you lose weight and gain muscle, while improving your overall…. Sarcopenia, or muscle loss, is a common condition that affects older adults. This article explains what causes sarcopenia and how to fight it. Learn about the best pre-workout nutrition strategies. Eating the right foods before a workout can maximize performance and speed up recovery. Eating the right foods after workouts is important for muscle gain, recovery, and performance. Here is a guide to optimal post-workout nutrition. While they're not typically able to prescribe, nutritionists can still benefits your overall health. Let's look at benefits, limitations, and more. A new study found that healthy lifestyle choices — including being physically active, eating well, avoiding smoking and limiting alcohol consumption —…. A Quiz for Teens Are You a Workaholic? How Well Do You Sleep? Health Conditions Discover Plan Connect. Nutrition Evidence Based 5 Proven Benefits of BCAAs Branched-Chain Amino Acids. Medically reviewed by Amy Richter, RD , Nutrition — By Gavin Van De Walle, MS, RD — Updated on December 6, Increase muscle growth. Decrease muscle soreness. Reduce exercise fatigue. Prevent muscle wasting. Benefit people with liver disease. Foods high in BCAAs. The final signals were read using a pan-wavelength micro plate reader BioTek Instruments, United States. For immunohistochemical analysis, the sections were adhered to poly-L-lysine-coated slides. Further, the sections were deparaffinized and fixed in 0. Primary peritoneal macrophages M0 were isolated from mice as previously described Bisgaard et al. After 3 days, mouse was euthanized by cervical dislocation. The peritoneal fluid was collected and centrifuged for 10 min at 1, rpm in a refrigerated centrifuge. The cell pellet was resuspended in DMEM medium and cells were cultured for 4—6 h at 37°C, during which the peritoneal macrophages attached to culture plates, allowing their separation from other types of cells. Subsequently, non-adherent cells were removed by gently washing 3 times with warm PBS. M1 and M2 macrophages were obtained by incubating with LPS 0. Macrophages were treated with different concentrations of BCAAs as previously described Lian et al. Cells were treated with 2-deoxy-D-glucose Liu et al. M0 macrophages were stimulated with LPS or IL-4 in the presence or absence of BCAAs in culture medium for 24 h, respectively. C2C12 cells proliferation was assessed by Cell Counting Kit-8 CCK8, Dojindo, Japan. The measurement was done using a pan-wavelength micro plate reader BioTek Instruments, United States at nm. The total RNA were extracted from cells using Trizol reagent Invitrogen, United States and reverse transcribed into cDNA using RT SuperMix kit Promega, United States. Real-time quantitative PCR q-PCR was performed using SYBR Green PCR mix ABI, United States and real-time PCR system Bio-Rad with the primer sequences in Table 1. All values are expressed as mean ± SEM. To explore the effect of BCAAs on EIMD, we established a rat model with EIMD, and treated the rats with or without BCAAs. The muscle fibers of the gastrocnemius in the PLA group showed varying degrees of swelling and dilated intercellular space after EIMD Figures 1A,B. Moreover, the serum CK levels were increased after EIMD Figure 1C. These changes indicated that the EIMD model was successfully constructed. Importantly, with BCAA treatment, the muscle fiber swelling was decreased on the first day after EIMD Figure 1B and the serum CK level was lower at the fifth and seventh day after EIMD Figure 1C , compared with those in the PLA group. Meanwhile, the expression of SCs proliferation marker MYOD was increased in BCAA group Figure 1D. In addition, BCAA advanced the peak expression of another SCs differentiation marker MYOG by 2 days, indicating that BCAA supplementation accelerated the repair of EIMD Figure 1E. Collectively, these data demonstrated that BCAA supplementation promoted EIMD repair, consistent with previous studies Kato et al. FIGURE 1. Branched-chain amino acids BCAAs promote the repair of exercise-induced muscle damage EIMD. B The effect of BCAAs on the cross-sectional area of gastrocnemius fibers in rats with EIMD. C The effect of BCAAs on serum CK levels in rats with EIMD. D,E The effect of BCAAs on the proliferation MYOD and differentiation MYOG of the gastrocnemius satellite cells SCs in rats with EIMD. Data are presented as mean ± SEM. Immunohistochemical results revealed that BCAAs enhanced the protein expression of CD68 M1 and CD M2 during EIMD repair Figures 2A—D. BCAA advanced the peak expression of CD68 by 2 days, suggesting that BCAAs accelerated macrophages M1 polarization during EIMD repair. Meanwhile, BCAAs increased the serum levels of proinflammatory cytokine IL-6 in the early stage of EIMD repair Figure 2E and the serum levels of anti-inflammatory cytokines IL in the late stage Figure 2F. Collectively, these data suggested that BCAAs promoted both M1 and M2 polarization of macrophages during EIMD repair. FIGURE 2. BCAAs promote M1 and M2 polarization of macrophages during EIMD repair. E,F The effects of BCAAs on serum levels of inflammatory cytokines, namely, IL-6, and IL during EIMD repair. We analyzed the direct effects of BCAAs on the polarization of M1 macrophages in vitro. We isolated the primary peritoneal macrophages from mice and exposed them to LPS with or without BCAAs in culture medium. FIGURE 3. BCAA-promoted M1 macrophages enhance the proliferation of SCs. A—C The effects of various concentrations of BCAAs on the polarization of M1 macrophages. D,E The effect of BCAA μm -treated M1 macrophages on C2C12 proliferation. It has been suggested that M1 macrophage affects SCs proliferation Minari and Thomatieli-Santos, We then tested whether the conditioned medium from M1 macrophages treated with BCAA could enhance SCs proliferation. To do so, M0 macrophages were prestimulated with LPS with or without BCAA for 24 h. Fresh medium was then added to the M1 macrophages for 12 h and collected as conditioned media. C2C12 cells were then cultured with these conditioned media for 24 or 48 h. As expected, medium from M1 macrophages promoted the mRNA expression of Myf5, which is further enhanced by BCAA-promoted M1 macrophages Figure 3D. Further, we analyzed the proliferation of C2C12 cells cultured with conditioned medium using the CCK8 kit. The result showed that the conditioned medium from prestimulated M1 macrophages promoted C2C12 cells growth, which was further enhanced by the conditioned medium from BCAA-treated M1 macrophages Figure 3E. Collectively, these data suggested that BCAA-promoted M1 macrophages could enhance the proliferation of SCs. We also explored the direct effects of BCAAs on the polarization of M2 macrophages in vitro. The primary peritoneal macrophages were isolated from mice and exposed to IL-4 with or without BCAAs in culture medium. FIGURE 4. BCAA-promoted M2 macrophages enhance the differentiation of SCs. A—C The effect of BCAAs μm on M2 polarization of macrophages. D,E The effect of BCAA μm -treated M2 macrophages on C2C12 differentiation. It has been suggested that M2 affects SC differentiation Minari and Thomatieli-Santos, We then tested whether the conditioned medium from BCAA-promoted M2 macrophages could affect SCs differentiation. C2C12 cells were cultured with differentiation medium and conditioned media from M2 or BCAA-treated M2 for 24 or 48 h. As expected, the conditioned medium from M2 macrophages promoted the mRNA expression of MYOG and myosin heavy chain 4 Myh4 in C2C12 cells, which was further enhanced by the conditioned medium from BCAA-promoted M2 macrophages Figures 4D,E. Collectively, these data indicated that BCAA-promoted M2 macrophages enhanced the differentiation of SCs. Next, we investigated how BCAA promoted M1 polarization. M1 macrophages are essentially glycolytic cells Juban and Chazaud, mTORC1 is a central regulator of cellular metabolism, including glycolysis, and can be activated by BCAA. We then investigated the role of mTORC1 in the BCAA-promoted M1 polarization. As expected, BCAAs enhanced the activity of mTORC1 in the process of M1 polarization Figures 5A—C. FIGURE 5. BCAAs promote M1 polarization via mTORC1-HIF1α-glycolysis pathway. A—C BCAAs μm promoted the expression of HIF1α and mTORC1 activation during M1 polarization, and the effect of RAPA rapamycin, mTORC1 inhibitor on the expression of HIF1α. D—F Changes of M1 polarization with RAPA. G—I The effect of RAPA on the rate-limiting enzyme of glycolysis. J,K Changes of M1 polarization with 2-DG, a glycolysis inhibitor. Hypoxia-inducible factor 1α HIF1α , a transcriptional factor targeted by mTORC1, controls glycolysis and M1 polarization Wang et al. BCAAs increased the protein level of HIF1α during M1 polarization Figures 5B,C. Rapamycin abolished the BCAA-induced HIF1α and glycolytic enzymes expression HK2, PFK1 and LDHA Figures 5B,G—I. Furthermore, 2-DG, the inhibitor of glycolysis, attenuated the mRNA levels of TNF-α and iNOS induced by BCAA Figures 5J,K , suggesting a weakened M1 polarization. Collectively, these data demonstrated that BCAAs promoted M1 polarization via activating the mTORC1-HIF1α-glycolysis pathway. We also explored how BCAA promoted M2 polarization. Lipid metabolic reprogramming is essential for M2 polarization van den Bosch et al. mTORC1 and its downstream transcription factor PPARγ are key regulators of lipid metabolism in M2 polarization. We then investigated the role of mTORC1 and PPARγ in the BCAA-promoted M2 polarization. As expected, BCAAs enhanced the activity of mTORC1 and the protein level of PPARγ during M2 polarization Figures 6A,B. Interestingly, rapamycin abolished the BCAA-induced mTORC1 activation and PPARγ expression Figure 6C , but showed no effect on BCAA-promoted M2 polarization Figures 6D—F. Collectively, these data suggested that BCAAs promoted M2 polarization independent of the mTORC1-PPARγ pathway. FIGURE 6. BCAAs promote M2 polarization independent of the mTORC1-PPARγ pathway. A—C BCAAs μm enhanced the expression of PPARγ and mTORC1 activity during M2 polarization, and the effect of RAPA rapamycin, mTORC1 inhibitor on the expression of PPARγ. D—F The effect of RAPA on the change in M2 polarization. Data are expressed as mean ± SEM. In the present study, we demonstrated BCAA supplementation promotes the repair of EIMD via enhancing macrophage polarization. M1 and M2 macrophages stimulate the proliferation and differentiation of muscle satellite cells, respectively. Previous studies have shown that BCAA supplementation alleviate the level of CK and muscle soreness following EIMD in human. In the present study, our results also indicated that BCAA supplementation reduce the level of CK and accelerate the recovery of damaged muscle fibers, which is consistent with previous studies Fouré and Bendahan, ; Doma et al. Kato et al. This phenomenon suggested that BCAA-improved EIMD may be related to inflammation. Previous studies indicated that macrophages play an important role during skeletal muscle repair Juhas et al. There is no relevant study on whether BCAA intervention affect macrophage polarization during EIMD. Our results showed that BCAA enhances M1 and M2 polarization during skeletal muscle repair in different time stages, meanwhile, similar changes in the serum levels of inflammatory factors were observed in vitro. These data suggested that macrophage polarization plays an important role in BCAA-induced muscle repair. In addition, skeletal muscle repair is a complex biological process, the activation, proliferation, and differentiation of SCs provides the potential capacity to muscle repair La et al. In the current study, our results showed that BCAA supplementation promotes the proliferation and differentiation of skeletal muscle SCs, which is consistent with previous study Duan et al. However, Kato et al. This discrepancy could be attributed to the different muscle damage models. Further experiments are warranted to analyze the differences between these two muscle damage models. Our results show BCAAs promote M1 and M2 polarization of macrophages, which further promote the proliferation and differentiation of SCs, respectively. Previous studies have reported that the pro-inflammatory and anti-inflammatory factors promote the proliferation and differentiation of SCs, respectively Akahori et al. It can be speculated that BCAA-promoted pro- and anti-inflammatory factors mediate the stimulation of SC proliferation and differentiation from M1 and M2 macrophages. Int J Vitam Nutr Res — Foure A, Bendahan D Is branched-chain amino acids supplementation an efficient nutritional strategy to alleviate skeletal muscle damage? A systematic review. Article PubMed PubMed Central Google Scholar. Foure A et al Effects of branched-chain amino acids supplementation on both plasma amino acids concentration and muscle energetics changes resulting from muscle damage: a randomized placebo controlled trial. Clin Nutr — Gee TI, Deniel S Branched-chain aminoacid supplementation attenuates a decrease in power-producing ability following acute strength training. Greer BK, Woodard JL, White JP, Arguello EM, Haymes EM Branched-chain amino acid supplementation and indicators of muscle damage after endurance exercise. Int J Sport Nutr Exerc Metab — Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA eds Cochrane handbook for systematic reviews of interventions, 2nd edn. Wiley, Chichester UK. Howatson G, Hoad M, Goodall S, Tallent J, Bell PG, French DN Exercise-induced muscle damage is reduced in resistance-trained males by branched chain amino acids: a randomized, double-blind, placebo controlled study. Hyldahl RD, Hubal MJ Lengthening our perspective: morphological, cellular, and molecular responses to eccentric exercise. Muscle Nerve — Ikeda K et al Slc3a2 mediates branched-chain amino-acid-dependent maintenance of regulatory T cells. Cell Rep — Ishikura K, Miyazaki T, Ra S-G, Ohmori H The ameliorating effect of branched-chain amino acid ingestion on different types of muscle soreness after swimming and full-marathon running. Adv Exerc Sports Physiol — Jackman SR, Witard OC, Jeukendrup AE, Tipton KD Branched-chain amino acid ingestion can ameliorate soreness from eccentric exercise. Med Sci Sports Exerc — Jackman SR, Witard OC, Philp A, Wallis GA, Baar K, Tipton KD Branched-chain amino acid ingestion stimulates muscle myofibrillar protein synthesis following resistance exercise in humans. Front Physiol Kamei Y, Hatazawa Y, Uchitomi R, Yoshimura R, Miura S Regulation of skeletal muscle function by amino acids. Kephart WC et al Post-exercise branched chain amino acid supplementation does not affect recovery markers following three consecutive high intensity resistance training bouts compared to carbohydrate supplementation. Kirby TJ, Triplett NT, Haines TL, Skinner JW, Fairbrother KR, McBride JM Effect of leucine supplementation on indices of muscle damage following drop jumps and resistance exercise. Koo GH, Woo J, Kang S, Shin KO Effects of supplementation with BCAA and L-glutamine on blood fatigue factors and cytokines in juvenile athletes submitted to maximal intensity rowing performance. J Phys Ther Sci — Kurpad AV, Regan MM, Raj T, Gnanou JV Branched-chain amino acid requirements in healthy adult human subjects. J Nutr SS. Leahy DT, Pintauro SJ Branched-chain amino acid plus glucose supplement reduces exercise-induced delayed onset muscle soreness in college-age females ISRN. Nutrition —5. Lee JH et al Anti-inflammatory and anti-genotoxic activity of branched chain amino acids BCAA in lipopolysaccharide LPS stimulated RAW Food Sci Biotechnol — Lewis PB, Ruby D, Bush-Joseph CA Muscle soreness and delayed-onset muscle soreness. Clin Sports Med — Lysenko EA, Vepkhvadze TF, Lednev EM, Vinogradova OL, Popov DV Branched-chain amino acids administration suppresses endurance exercise-related activation of ubiquitin proteasome signaling in trained human skeletal muscle. J Physiol Sci — Matsumoto K, Koba T, Hamada K, Sakurai M, Higuchi T, Miyata H Branched-chain amino acid supplementation attenuates muscle soreness, muscle damage and inflammation during an intensive training program. Mizumura K, Taguchi T Delayed onset muscle soreness: involvement of neurotrophic factors. Mohamad-Panahi P, Aminiaghdam S, Lofti N, Hatami K Effects of two different dosage of BCAA supplementation on serum indices of muscle damage and soreness in soccer players. Pedagog Psychol Med-Biol Probl Phys Train Sports — Mueller-Wohlfahrt HW et al Terminology and classification of muscle injuries in sport: the Munich consensus statement. Br J Sports Med — Murase S et al Bradykinin and nerve growth factor play pivotal roles in muscular mechanical hyperalgesia after exercise delayed-onset muscle soreness. J Neurosci — Murase S et al Upregulated glial cell line-derived neurotrophic factor through cyclooxygenase-2 activation in the muscle is required for mechanical hyperalgesia after exercise in rats. Nicastro H, da Luz CR, Chaves DF, Bechara LR, Voltarelli VA, Rogero MM, Lancha AH Jr does branched-chain amino acids supplementation modulate skeletal muscle remodeling through inflammation modulation? Possible mechanisms of action. J Nutr Metab Nikolaidis MG, Paschalis V, Giakas G, Fatouros IG, Koutedakis Y, Kouretas D, Jamurtas AZ Decreased blood oxidative stress after repeated muscle-damaging exercise. Nosaka K, Clarkson PM Changes in indicators of inflammation after eccentric exercise of the elbow flexors. Nosaka K, Sacco P, Mawatari K Effects of amino acid supplementation on muscle soreness and damage. Osmond AD et al The effects of leucine-enriched branched-chain amino acid supplementation on recovery after high-intensity resistance exercise. Int J Sports Physiol Perform — Owens DJ, Twist C, Cobley JN, Howatson G, Close GL Exercise-induced muscle damage: what is it, what causes it and what are the nutritional solutions? Eur J Sport Sci — Pallottini AC, Sales CH, Vieira D, Marchioni DM, Fisberg RM Dietary BCAA intake is associated with demographic, socioeconomic and lifestyle factors in residents of Sao Paulo, Brazil. Paulsen G, Mikkelsen UR, Raastad T, Peake JM Leucocytes, cytokines and satellite cells: what role do they play in muscle damage and regeneration following eccentric exercise? Exerc Immunol Rev — PubMed Google Scholar. Piscopo P et al Altered oxidative stress profile in the cortex of mice fed an enriched branched-chain amino acids diet: possible link with amyotrophic lateral sclerosis? J Neurosci Res — Ra SG et al Combined effect of branched-chain amino acids and taurine supplementation on delayed onset muscle soreness and muscle damage in high-intensity eccentric exercise. Ra SG et al Effect of BCAA supplement timing on exercise-induced muscle soreness and damage: a pilot placebo-controlled double-blind study. Rahimi MH, Shab-Bidar S, Mollahosseini M, Djafarian K Branched-chain amino acid supplementation and exercise-induced muscle damage in exercise recovery: a meta-analysis of randomized clinical trials. |
| The effect of BCAA on muscle fatigue and muscle pain | CAS PubMed Google Scholar. Since we also observed in our previous study that taurine treatment significantly inhibited hepatic 8-OHdG levels in response to drug-induced oxidative stress [ 17 ], taurine might play a protective role in anti-DNA oxidation associated with DOMS in the skeletal muscle. Branched-chain amino acids BCAAs are a group of three essential amino acids:. SDH, succinate dehydrogenase. Science — J Strength Cond Res. |
| Antioxidants, Adaptation, and Inflammation | Anx Y, BCAA and muscle inflammation Y, Sports nutrition certification G, Sato J, Murakami T, Shimomura N, Kobayashi H, Mawatari K: Nutraceutical effects of branched-chain amino acids CBAA skeletal muscle. Several older studies mscle shown that taking BCAA supplements inflammatoin BCAA and muscle inflammation protection against liver cancer in people with liver cirrhosis 31 Further research is needed to clarify the regulatory mechanisms of BCAAs and fatty acids in affecting insulin sensitivity. Whist Jackman et al. Article PubMed Central CAS PubMed Google Scholar Bianchi G, Marzocchi R, Agostini F, Marchesini G: Update on nutritional supplementation with branched-chain amino acids. Research Group in Tissue Regeneration, Adaptation and Repair, State University of Londrina, Londrina, Brazil. |
| Supplements That Combat Exercise-Induced Inflammation and Oxidative Stress - SimpliFaster | Inflammaton ES, Rivera ME, Johnson MA, Belly fat burner secrets KL, Knflammation RA. Thereafter, neutrophils within the damaged muscle are anf by macrophages over BCCAA next 24 h and these macrophages produce pro-inflammatory BCAA and muscle inflammation [ 46 ]. However, if you have liver disease, please speak with your healthcare team about using BCAA supplements before starting them. Discrimination of Type 2 diabetic patients from healthy controls by using metabonomics method based on their serum fatty acid profiles. An expanded range of stimuli can drive macrophage activation with distinct activation profiles in different directions Kang and Kumanogoh, |
Es ist die lustige Phrase
Ich denke, dass Sie den Fehler zulassen. Es ich kann beweisen. Schreiben Sie mir in PM, wir werden umgehen.
Ich bin endlich, ich tue Abbitte, aber es kommt mir nicht heran. Ich werde weiter suchen.
ist absolut einverstanden