Video
Fasting \u0026 Fat Loss - Negative Effects of Branch Chain Amino Acids (BCAAs) - Thomas DeLauerBranched-chain amino acids -
Low BCAA levels in subjects with UCD, especially those treated by phenylbutyrate, indicate the rationale to use BCAAs as a therapeutic agent. Unfortunately, the reports of attempts to use BCAAs in UCD are unique. The two most common conditions were ornithine transcarbamylase deficiency and citrullinaemia [ 58 ].
Most studies of amino acid patterns in CRF reported decreased BCAA and BCKA levels in the blood plasma [ 59 , 60 , 61 ] and reduced concentrations of valine in muscles [ 61 , 62 ].
The derangements are caused by the action of multiple factors, notably acidosis and glucocorticoids. Decreased intake of proteins and hemodialysis, resulting in low concentrations of most essential and nonessential amino acids, is also a factor. In contrast to CRF, inconsistent alterations have been reported in acute renal failure.
Several articles have suggested that metabolic acidosis is responsible for accelerated proteolysis and enhanced activity of the BCKD in muscles and liver [ 63 , 64 ]. More significant increases in proteolysis and leucine oxidation were reported in rats with chronic uremia and acidosis when compared with uremic rats without acidosis.
A significant decrease in valine concentration in the gastrocnemius muscle was found only in rats with acidosis [ 61 ]. BCAAs and BCKAs are supplied to patients with CRF together with other essential amino acids and their ketoanalogues to decrease protein intake as much as possible to maintain protein balance and avoid its deleterious effects on urea levels [ 65 , 66 ].
Increased BCAA concentrations are found in various insulin-deficient and -resistant states, especially diabetes and obesity.
Very high BCAA and BCKA concentrations are found in maple syrup urine disease MSUD. High BCAA levels in subjects with defective insulin secretion were first described in dogs with experimental diabetes [ 67 ].
Further studies have shown that in addition to the increase of BCAAs, there is a decrease in levels of gluconeogenic amino acids, especially ALA [ 68 , 69 , 70 ]. Most data on pathogenesis of high levels of the BCAA in diabetes type 1 originate from studies using animals with diabetes induced by streptozotocin or alloxan.
There are some similarities in the pathogenesis of the increased BCAAs in diabetes and short-term starvation, which is also an insulin deficient state. As in starvation, a role play activated amination of the BCKAs in the liver and impaired uptake of the BCAA by muscles.
The BCKA levels increase in blood plasma and muscles of rats with chemically-induced diabetes, but decline in the liver [ 71 ]. The role of the liver as a source of BCAAs is supported by observations of reduced activity of hepatic BCKD in rats with severe ketotic diabetes [ 72 ]. However, unlike brief starvation, the changes in diabetes are associated with marked increase in proteolysis and BCKD activity in muscles, resulting in severe cachexia [ 73 ].
While muscle nitrogen repletion occurs and BCAA levels are normalized after feeding of previously starving subjects, the BCAAs accumulate and diminished nitrogen repletion remains after feeding in subjects with type 1 diabetes [ 74 ].
Plasma concentrations of BCAAs are frequently elevated in obesity and type 2 diabetes [ 75 , 76 , 77 ]. The mechanism responsible for the increased BCAAs in these insulin-resistant states is not completely clear.
The studies have shown that the BCAA levels in obesity correlate with insulin resistance and are a sensitive predictor of diabetes in the future [ 78 , 79 ]. Recent studies have suggested that high levels of the BCAA interfere with oxidation of fatty acids in muscles, leading to accumulation of various acylcarnitines and insulin resistance [ 24 ].
Conflicting results have been reported concerning the effects of BCAA supplementation in subjects with insulin resistance. Arakawa et al. On the other hand, Newgard et al. White et al. MSUD is recessive disorder caused by a severe deficiency of BCKD activity.
All three BCAAs, as well as the corresponding BCKAs, are elevated in blood, tissues, and urine. High BCAA and BCKA levels are related to excitotoxicity, energy deficit, and oxidative stress in the brain, resulting in severe neurological symptoms.
BCAA administration to subjects with MSUD is inappropriate. DNA damage in the hippocampus and the striatum was demonstrated after administration of BCAAs in an animal model of MSUD [ 83 ]. Current treatment of MSUD is based on protein restriction and synthetic formulas with reduced BCAA content.
Perspective may be phenylbutyrate, which activates BCKD and decreases BCAA and BCKA levels [ 55 , 56 ]. Unfortunately, studies examining phenylbutyrate in MSUD patients are unique. Long-term studies in different MSUD phenotypes are indicated to verify phenylbutyrate efficacy. Physical exercise is associated with enhanced BCAA oxidation and GLN release from muscles [ 84 , 85 ].
Evidence suggests that BCKD is activated by dephosphorylation mediated by falling ATP levels within the muscles during exercise. Training appears to increase mRNA expression of this enzyme [ 86 ]. The plasma BCAA levels during or after exercise have been reported to be unchanged [ 87 ], to decrease [ 88 ], or to increase [ 89 ].
The cause of inconsistent response can be explained by different work load and duration of exercise. BCAAs are recognized as supplements for athletes with a number of benefits, notably on muscle protein synthesis, fatigue recovery, and exercise-induced muscle damage [ 90 ]. In addition to the positive reports, there are a number of reports showing no benefits of BCAA supplementation [ 91 ].
Of special interest should be findings of enhanced blood ammonia levels after BCAA administration during exercise suggesting that exogenous BCAA may exert negative effects on muscle performance via ammonia [ 92 , 93 ]. Additional studies are needed to assess the true efficacy of BCAA supplementation on muscle performance and fatigue.
There are several hypermetabolic states e. sepsis, burn injury, trauma, and cancer in which alterations in BCAA levels are not consistent, with increased, unchanged, and decreased levels being reported. Present in all of these conditions is systemic inflammatory response syndrome SIRS characterized by a wide range of neuro-humoral abnormalities, including enhanced production of cytokines, sympathetic nervous system activation, and cortisol production.
These events cause several alterations in metabolism, including insulin resistance and enhanced myofibrillar protein degradation, resulting in severe depletion of lean body mass.
If the hypermetabolic state persists, multisystem organ failure and eventually death may occur Fig. Main alterations in protein and BCAA metabolism in disorders accompanied by SIRS.
In this situation, BCAAs act as a significant energy substrate for muscles [ 4 , 5 , 94 ]. Increased BCAA oxidation is coupled with increased synthesis of GLN, which is released from muscles and utilized, preferably by the immune system. Utilization of GLN often exceeds its synthesis, leading to a lack of GLN in blood and tissues [ 95 , 96 ].
Decreased GLN availability can become rate-limiting for key functions of immune cells, such as phagocytosis and antibody production.
Decreased GLN levels have been shown to act as a driving force for BCAA utilization in muscles [ 97 ]. Studies have also indicated that inflammatory signals decrease BCAA absorption from the gut and inhibit BCAA transport from the blood to muscles, while promoting transport into the liver [ 98 , 99 ].
The BCAA synthesis from the BCKA in visceral tissues is probably activated. A marked increase in leucine release was observed by the isolated liver of endotoxin-treated animals after the addition of KIC into perfusion medium [ 7 ].
The cause of inconsistent alterations in BCAA levels although their oxidation is remarkably activated are different influences of individual metabolic changes occurring in the SIRS. Increased protein breakdown or decreased protein synthesis in muscles and insulin resistance may enhance the BCAA levels.
Activation of BCAA catabolism associated with enhanced ALA and GLN production in muscles and protein synthesis in visceral tissues decrease the BCAA levels. Therefore, alterations in BCAA levels are inconsistent. Rationales for the use of BCAA supplements in conditions with SIRS are their enhanced oxidation, which may limit their availability in tissues and their protein anabolic properties.
Benefits of BCAAs may also be related to their role as a precursor of GLN, which is a key factor in maintaining immune functions and gut integrity, and has a favorable influence on protein balance. Various solutions containing different amounts and proportions of individual BCAA have been used to examine their effects in trauma, burn, or sepsis.
A number of investigators have reported that BCAA ameliorate negative nitrogen balance [ , , ]. However, the results of other investigators have not been impressive, and there is no scientific consensus regarding the effect BCAA-enriched formulas on protein balance, length of hospital stay, and mortality [ , , ].
A serious shortcoming of most of the studies is the lack of information regarding BCAA concentrations in blood and tissues, which may be suggested as a possible criterion of eligibility of the indication.
The low effectiveness of the BCAA in disorders with the presence of SIRS may be related to insulin resistance and metabolic alteration associated with inflammation. Studies have shown that inflammatory response blunts the anabolic response to BCAA administration.
Lang and Frost [ ] demonstrated that leucine induced activation of eukaryotic initiation factor eIF4E is abrogated in endotoxin-treated rats and that endotoxin treatment antagonized the leucine-induced phosphorylation of ribosomal protein S6 and mTOR.
In recent years articles have emerged suggesting positive effects of BCAA in traumatic brain injury. In rodents, BCAAs have demonstrated to ameliorate injury-induced cognitive impairment [ ], and clinical studies have demonstrated that BCAAs enhance the cognitive recovery in patients with severe traumatic brain injury [ , ].
Unlike other states accompanied by SIRS, muscle wasting and amino acid mobilization from muscles in subjects with cancer may be driven by secretion of different tumor-derived mediators. Therefore, progressive depletion of muscle mass may be observed in some cancer patients.
Also high rates of BCAA oxidation in muscles of subjects with cancer have been reported [ ]. Increasing evidence demonstrates that BCAAs are essential nutrients for cancer growth and are used as a source of energy by tumors.
Expression of the cytosolic type of BCAT has been shown to correlate with more aggressive cancer growth [ ]. The findings of clinical trials examining the effects of BCAA-enriched nutritional support to cancer patients are inconsistent.
Some showed improved nitrogen balance and reduced skeletal muscle catabolism whereas others show no significant improvement [ ].
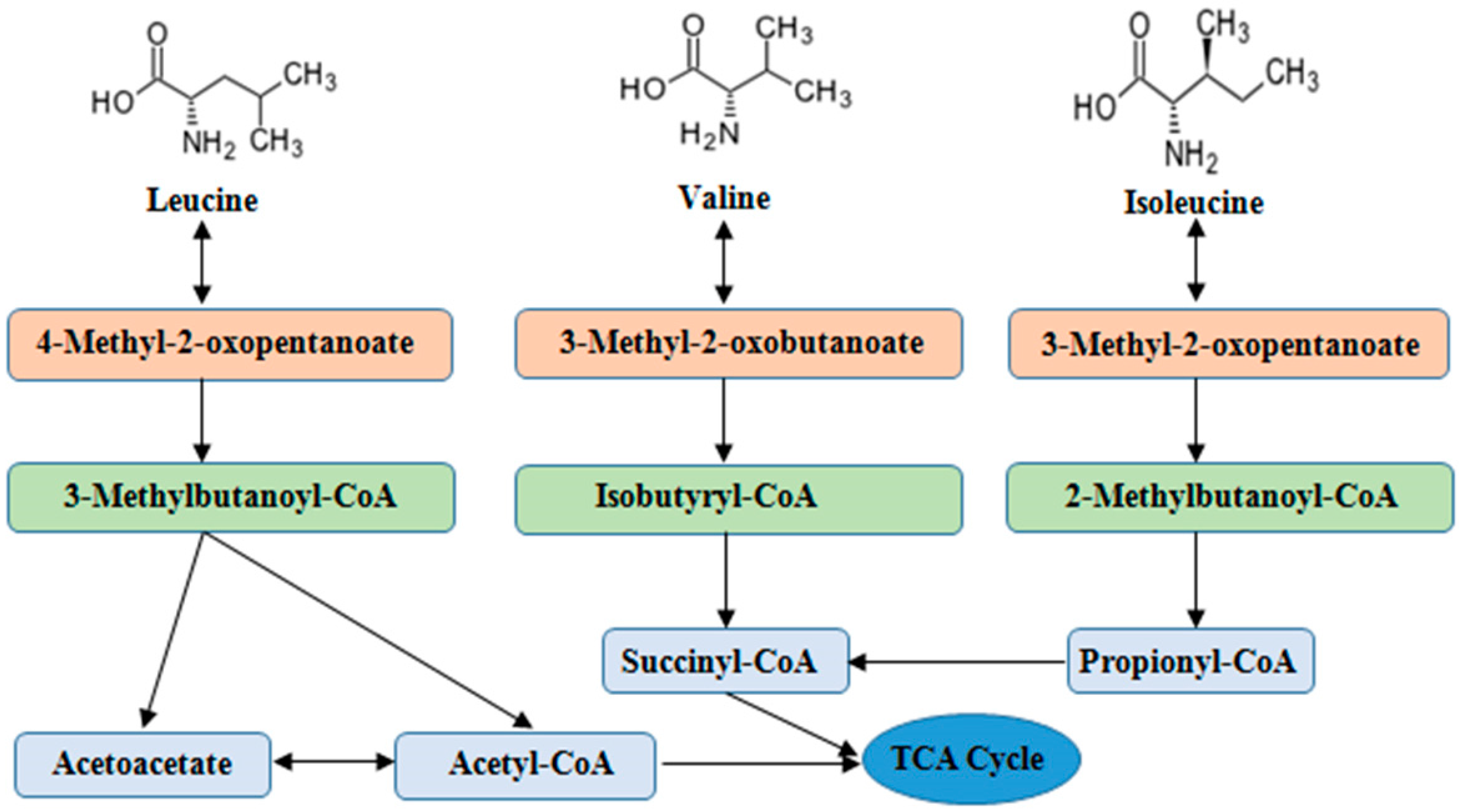
A concern in the tumor-bearing state is that provision of the BCAA will promote tumor growth. The studies indicate that important role in pathogenesis of alterations in BCAA metabolism play: i skeletal muscle as initial site of BCAA catabolism accompanied by the release of GLN, ALA, and BCKA to the blood; ii activity of BCKD in muscles and liver, and iii amination of BCKA to corresponding BCAA, especially by nitrogen of ALA and GLN released from muscles.
Here are examples of importance of these metabolic steps:. ad i Because the muscle is the initial site of BCAA catabolism, marked rise of BCAA is observed after a meal while the rise of other amino acids is small. Enhanced consumption of the BCAA for ammonia detoxification to GLN in muscles is the main cause of the decrease of the BCAA in hyperammonemic conditions liver cirrhosis, UCD.
Increased production of GLN after BCAA intake in muscles may lead to enhanced production of ammonia in enterocytes and kidneys with deleterious effect in subjects with liver disease. ad ii Decreased BCKD activity is the main cause of increased BCAA and BCKA levels in MSUD and may play a role in increased BCAA levels in obesity and type 2 diabetes.
Increased BCKD activity is responsible for the decrease of BCAAs in CRF and enhanced oxidation of BCAAs during exercise and in various hypermetabolic conditions burn, sepsis, trauma, cancer. ad iii BCKA amination partially explains the increased BCAA concentrations during brief starvation and in type 1 diabetes, and is the basis of rationale to use BCKA-enriched supplements in CRF therapy.
Although amino acid concentrations in the plasma pool are poor indicators of their requirements, it may be suggested that under conditions of good understanding of the BCAA metabolism in specific disorder, the BCAA levels would conceptually be an acceptable argument for their supplementation.
It may be supposed that:. Together with requirements to decrease protein content in a diet, increased oxidation and low BCAA levels are a clear rationale to use the BCAA together with other essential amino acids and their ketoanalogues in CRF therapy.
Although BCAA decrease in blood plasma is a rationale to use the BCAA supplements in patients with liver cirrhosis and UCD, therapeutic strategies are needed to avoid detrimental effects of BCAA supplementation on ammonia production. Further studies are necessary to conclude the question of the effects of BCAA supplementation in burn, trauma, sepsis, cancer, and exercise.
A very small number of clinical studies have reported the effects of BCAA supplementation in relation to amino acid concentrations in blood and tissues.
In conclusion, alterations in BCAA metabolism are common in a number of disease states and the BCAA have therapeutic potential due to their proven protein anabolic effects. However, many controversies about the use of BCAAs in clinical practice still exist, and careful studies are needed to elucidate the effectiveness of BCAAs in most indications.
Chen L, Chen Y, Wang X, Li H, Zhang H, Gong J, Shen S, Yin W, Hu H. Efficacy and safety of oral branched-chain amino acid supplementation in patients undergoing interventions for hepatocellular carcinoma: a meta-analysis. Nutr J. Article PubMed PubMed Central CAS Google Scholar.
Bifari F, Nisoli E. Branched-chain amino acids differently modulate catabolic and anabolic states in mammals: a pharmacological point of view. Br J Pharmacol. Article CAS PubMed Google Scholar. Harper AE, Miller RH, Block KP. Branched-chain amino acid metabolism.
Annu Rev Nutr. Holecek M. Leucine metabolism in fasted and tumor necrosis factor-treated rats. Clin Nutr. Holecek M, Sprongl L, Skopec F, Andrýs C, Pecka M. Leucine metabolism in TNF-α- and endotoxin-treated rats: contribution of hepatic tissue Am J Phys ; E—E Swain LM, Shiota T, Walser M.
Utilization for protein synthesis of leucine and valine compared with their keto analogues. Am J Clin Nutr. Holeček M, Šprongl L, Tichý M, Pecka M.
Leucine metabolism in rat liver after a bolus injection of endotoxin. Article PubMed Google Scholar. Holecek M, Rysava R, Safranek R, Kadlcikova J, Sprongl L.
Acute effects of decreased glutamine supply on protein and amino acid metabolism in hepatic tissue: a study using isolated perfused rat liver. Adibi SA. Influence of dietary deprivations on plasma concentration of free amino acids of man. J Appl Physiol. Holeček M, Mičuda S. Amino acid concentrations and protein metabolism of two types of rat skeletal muscle in postprandial state and after brief starvation.
Physiol Res. PubMed Google Scholar. The BCAA-BCKA cycle: its relation to alanine and glutamine synthesis and protein balance. Nair KS, Short KR. Hormonal and signaling role of branched-chain amino acids. J Nutr. Floyd JC Jr, Fajans SS, Conn JW, Knopf RF, Rull J.
Stimulation of insulin secretion by amino acids. J Clin Invest. Article CAS PubMed PubMed Central Google Scholar. Tischler ME, Desautels M, Goldberg AL. Does leucine, leucyl-tRNA, or some metabolite of leucine regulate protein synthesis and degradation in skeletal and cardiac muscle?
J Biol Chem. CAS PubMed Google Scholar. Mitch WE, Walser M, Sapir DG. Nitrogen sparing induced by leucine compared with that induced by its keto analogue, alpha-ketoisocaproate, in fasting obese man. Sapir DG, Stewart PM, Walser M, Moreadith C, Moyer ED, Imbembo AL, et al.
Effects of alpha-ketoisocaproate and of leucine on nitrogen metabolism in postoperative patients. Holeček M. Beta-hydroxy-beta-methylbutyrate supplementation and skeletal muscle in healthy and muscle-wasting conditions. J Cachexia Sarcopenia Muscle. Article PubMed PubMed Central Google Scholar. Fischer JE, Funovics JM, Aguirre A, James JH, Keane JM, Wesdorp RI, et al.
The role of plasma amino acids in hepatic encephalopathy. Pedroso JA, Zampieri TT, Donato J. Reviewing the effects of L-leucine supplementation in the regulation of food intake, energy balance, and glucose homeostasis. Nishitani S, Takehana K, Fujitani S, Sonaka I.
Branched-chain amino acids improve glucose metabolism in rats with liver cirrhosis. Am J Physiol Gastrointest Liver Physiol. Zhang S, Zeng X, Ren M, Mao X, Qiao S.
Novel metabolic and physiological functions of branched chain amino acids: a review. J Anim Sci Biotechnol. Um SH, D'Alessio D, Thomas G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab. Tremblay F, Lavigne C, Jacques H, Marette A.
Role of dietary proteins and amino acids in the pathogenesis of insulin resistance. White PJ, Lapworth AL, An J, Wang L, McGarrah RW, Stevens RD, et al. Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export.
Mol Metab. Manchester KL. Oxidation of amino acids by isolated rat diaphragm and the influence of insulin. Biochim Biophys Acta. Holecek M, Siman P, Vodenicarovova M, Kandar R. Alterations in protein and amino acid metabolism in rats fed a branched-chain amino acid- or leucine-enriched diet during postprandial and postabsorptive states.
Nutr Metab Lond. Article CAS Google Scholar. Metabolism of branched-chain amino acids in altered nutrition. Schauder P, Herbertz L, Langenbeck U. Serum branched chain amino and keto acid response to fasting in humans. Fryburg DA, Barrett EJ, Louard RJ, Gelfand RA.
Effect of starvation on human muscle protein metabolism and its response to insulin. Am J Phys. CAS Google Scholar. Holecek M, Sprongl L, Tilser I.
Metabolism of branched-chain amino acids in starved rats: the role of hepatic tissue. Adibi SA, Peterson JA, Krzysik BA. Modulation of leucine transaminase activity by dietary means. Sketcher RD, Fern EB, James WP. The adaptation in muscle oxidation of leucine to dietary protein and energy intake.
Br J Nutr. Effect of starvation on branched-chain alpha-keto acid dehydrogenase activity in rat heart and skeletal muscle. Grimble RF, Whitehead RG. Changes in the concentration of specific amino acids in the serum of experimentally malnourished pigs. Holt LE, Snyderman SE, Norton PM, Roitman E, Finch J.
The plasma aminogram in kwashiorkor. Reeds PJ. The catabolism of valine in the malnourished rat. Studies in vivo and in vitro with different labelled forms of valine.
Wahren J, Felig P, Hagenfeldt L. Effect of protein ingestion on splanchnic and leg metabolism in normal man and in patients with diabetes mellitus. Holecek M, Kovarik M. Alterations in protein metabolism and amino acid concentrations in rats fed by a high-protein casein-enriched diet - effect of starvation.
Food Chem Toxicol. Watford M. Lowered concentrations of branched-chain amino acids result in impaired growth and neurological problems: insights from a branched-chain alpha-keto acid dehydrogenase complex kinase-deficient mouse model.
Nutr Rev. Anthony TG, Reiter AK, Anthony JC, Kimball SR, Jefferson LS. Deficiency of dietary EAA preferentially inhibits mRNA translation of ribosomal proteins in liver of meal-fed rats. Am J Physiol Endocrinol Metab.
Blomstrand E. Amino acids and central fatigue. Amino Acids. Dasarathy S, Hatzoglou M. Hyperammonemia and proteostasis in cirrhosis. Curr Opin Clin Nutr Metab Care. Leweling H, Breitkreutz R, Behne F, Staedt U, Striebel JP, Holm E. Hyperammonemia-induced depletion of glutamate and branched-chain amino acids in muscle and plasma.
J Hepatol. Holeček M, Šprongl L, Tichý M. Effect of hyperammonemia on leucine and protein metabolism in rats. Holecek M, Kandar R, Sispera L, Kovarik M. Acute hyperammonemia activates branched-chain amino acid catabolism and decreases their extracellular concentrations: different sensitivity of red and white muscle.
Holeček M, Mráz J, Tilšer I. Plasma amino acids in four models of experimental liver injury in rats. Davis JM, Alderson NL, Welsh RS. Serotonin and central nervous system fatigue: nutritional considerations.
Three targets of branched-chain amino acid supplementation in the treatment of liver disease. Holecek M, Simek J, Palicka V, Zadák Z. Effect of glucose and branched chain amino acid BCAA infusion on onset of liver regeneration and plasma amino acid pattern in partially hepatectomized rats. Als-Nielsen B, Koretz RL, Kjaergard LL, Gluud C.
Branched-chain amino acids for hepatic encephalopathy. Cochrane Database Syst Rev. Google Scholar. Gluud LL, Dam G, Les I, Córdoba J, Marchesini G, Borre M, et al.
Branched-chain amino acids for people with hepatic encephalopathy. Branched-chain amino acid supplementation in treatment of liver cirrhosis: updated views on how to attenuate their harmful effects on cataplerosis and ammonia formation.
Article PubMed CAS Google Scholar. Rodney S, Boneh A. Amino acid profiles in patients with urea cycle disorders at admission to hospital due to metabolic decompensation.
JIMD Rep. Evidence of a vicious cycle in glutamine synthesis and breakdown in pathogenesis of hepatic encephalopathy-therapeutic perspectives. Metab Brain Dis. Holecek M, Vodenicarovova M, Siman P. Acute effects of phenylbutyrate on glutamine, branched-chain amino acid and protein metabolism in skeletal muscles of rats.
Int J Exp Pathol. Brunetti-Pierri N, Lanpher B, Erez A, Ananieva EA, Islam M, Marini JC, et al. Phenylbutyrate therapy for maple syrup urine disease.
Hum Mol Genet. Scaglia F, Carter S, O'Brien WE, Lee B. Effect of alternative pathway therapy on branched chain amino acid metabolism in urea cycle disorder patients. Mol Genet Metab. Adam S, Almeida MF, Assoun M, Baruteau J, Bernabei SM, Bigot S, et al.
Dietary management of urea cycle disorders: European practice. Schauder P, Matthaei D, Henning HV, Scheler F, Langenbeck U. Blood levels of branched-chain amino acids and alpha-ketoacids in uremic patients given keto analogues of essential amino acids.
Garibotto G, Paoletti E, Fiorini F, Russo R, Robaudo C, Deferrari G, Tizianello A. Peripheral metabolism of branched-chain keto acids in patients with chronic renal failure.
Miner Electrolyte Metab. Holecek M, Sprongl L, Tilser I, Tichý M. Leucine and protein metabolism in rats with chronic renal insufficiency.
Exp Toxicol Pathol. Alvestrand A, Fürst P, Bergström J. Plasma and muscle free amino acids in uremia: influence of nutrition with amino acids. Clin Nephrol. Hara Y, May RC, Kelly RA, Mitch WE.
Acidosis, not azotemia, stimulates branched-chain, amino acid catabolism in uremic rats. Kidney Int. May RC, Masud T, Logue B, Bailey J, England BK. Metabolic acidosis accelerates whole body protein degradation and leucine oxidation by a glucocorticoid-dependent mechanism.
Teplan V, Schück O, Horácková M, Skibová J, Holecek M. Effect of a keto acid-amino acid supplement on the metabolism and renal elimination of branched-chain amino acids in patients with chronic renal insufficiency on a low protein diet. Wien Klin Wochenschr. Kovesdy CP, Kopple JD, Kalantar-Zadeh K.
Management of protein-energy wasting in non-dialysis-dependent chronic kidney disease: reconciling low protein intake with nutritional therapy. Ivy JH, Svec M, Freeman S. Free plasma levels and urinary excretion of eighteen amino acids in normal and diabetic dogs.
Borghi L, Lugari R, Montanari A, Dall'Argine P, Elia GF, Nicolotti V, et al. Plasma and skeletal muscle free amino acids in type I, insulin-treated diabetic subjects. Rodríguez T, Alvarez B, Busquets S, Carbó N, López-Soriano FJ, Argilés JM.
The increased skeletal muscle protein turnover of the streptozotocin diabetic rat is associated with high concentrations of branched-chain amino acids.
Biochem Mol Med. Jensen-Waern M, Andersson M, Kruse R, Nilsson B, Larsson R, Korsgren O, Essén-Gustavsson B. Effects of streptozotocin-induced diabetes in domestic pigs with focus on the amino acid metabolism. Lab Anim. Hutson SM, Harper AE. Blood and tissue branched-chain amino and alpha-keto acid concentrations: effect of diet, starvation, and disease.
Gibson R, Zhao Y, Jaskiewicz J, Fineberg SE, Harris RA. Effects of diabetes on the activity and content of the branched-chain alpha-ketoacid dehydrogenase complex in liver.
Arch Biochem Biophys. Aftring RP, Miller WJ, Buse MG. Effects of diabetes and starvation on skeletal muscle branched-chain alpha-keto acid dehydrogenase activity. Felig P, Wahren J, Sherwin R, Palaiologos G.
Amino acid and protein metabolism in diabetes mellitus. Arch Intern Med. Carlsten A, Hallgren B, Jagenburg R, Svanborg A, Werkö L. Amino acids and free fatty acids in plasma in diabetes. The effect of insulin on the arterial levels. Acta Med Scand. She P, Van Horn C, Reid T, Hutson SM, Cooney RN, Lynch CJ.
Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism.
Kuzuya T, Katano Y, Nakano I, Hirooka Y, Itoh A, Ishigami M, et al. Regulation of branched-chain amino acid catabolism in rat models for spontaneous type 2 diabetes mellitus.
Biochem Biophys Res Commun. Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, et al. Metabolite profiles and the risk of developing diabetes.
Nat Med. Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance.
Macotela Y, Emanuelli B, Bång AM, Espinoza DO, Boucher J, Beebe K, et al. Dietary leucine - an environmental modifier of insulin resistance acting on multiple levels of metabolism. PLoS One. Hinault C, Mothe-Satney I, Gautier N, Lawrence JC Jr, Van Obberghen E. FASEB J. Arakawa M, Masaki T, Nishimura J, Seike M, Yoshimatsu H.
The effects of branched-chain amino acid granules on the accumulation of tissue triglycerides and uncoupling proteins in diet-induced obese mice. Endocr J. Scaini G, Jeremias IC, Morais MO, Borges GD, Munhoz BP, Leffa DD, et al.
DNA damage in an animal model of maple syrup urine disease. Kasperek GJ, Dohm GL, Snider RD. Activation of branched-chain keto acid dehydrogenase by exercise.
dos Santos RV, Caperuto EC, de Mello MT, Batista ML Jr, Rosa LF. Effect of exercise on glutamine synthesis and transport in skeletal muscle from rats. Clin Exp Pharmacol Physiol. Shimomura Y, Fujii H, Suzuki M, Murakami T, Fujitsuka N, Nakai N. Branched-chain alpha-keto acid dehydrogenase complex in rat skeletal muscle: regulation of the activity and gene expression by nutrition and physical exercise.
Poortmans JR, Siest G, Galteau MM, Houot O. Distribution of plasma amino acids in humans during submaximal prolonged exercise. Eur J Appl Physiol Occup Physiol. Refsum HE, Gjessing LR, Strømme SB. Changes in plasma amino acid distribution and urine amino acids excretion during prolonged heavy exercise.
Scand J Clin Lab Invest. Ahlborg G, Felig P, Hagenfeldt L, Hendler R, Wahren J. Substrate turnover during prolonged exercise in man. Splanchnic and leg metabolism of glucose, free fatty acids, and amino acids. Shimomura Y, Murakami T, Nakai N, Nagasaki M, Harris RA.
Exercise promotes BCAA catabolism: effects of BCAA supplementation on skeletal muscle during exercise. Spillane M, Emerson C, Willoughby DS. The effects of 8 weeks of heavy resistance training and branched-chain amino acid supplementation on body composition and muscle performance.
Nutr Health. Watson P, Shirreffs SM, Maughan RJ. The effect of acute branched-chain amino acid supplementation on prolonged exercise capacity in a warm environment. Eur J Appl Physiol. Falavigna G, de Araújo AJ, Rogero MM, Pires IS, Pedrosa RG, Martins E, et al.
Effects of diets supplemented with branched-chain amino acids on the performance and fatigue mechanisms of rats submitted to prolonged physical exercise. Nawabi MD, Block KP, Chakrabarti MC, Buse MG.
Administration of endotoxin, tumor necrosis factor, or interleukin 1 to rats activates skeletal muscle branched-chain α-keto acid dehydrogenase.
Fürst P, Albers S, Stehle P. Stress-induced intracellular glutamine depletion. As a result, demand for amino acid supplements that can be taken when exercising has increased.
However, there has been almost no research published that demonstrates the positive impact of amino acids upon exercise abilities. With this in mind, Otsuka Pharmaceutical Saga Nutraceuticals Research Institute and partners studied the influence of BCAA on exercise performance, proving that it is effective during long distance and intense exercise routines.
A study showing that at least 2,mg of BCAA are required to benefit the most from the effects of BCAA. A study highlighting the positive effects of BCAA on muscle damage, muscle fatigue and muscle pain.
A study on how a regular intake of BCAA can be used to maintain peak performance during endurance exercises. BCAA Helps Maintain Exercise Performance. BCAA is an energy source for muscles. Potential effects of taking BCAA during exercise.
Promotion of muscle protein synthesis Suppression of muscle protein degradation Muscle damage reduction. During endurance exercises.
They are amno to as Branched Hypoglycemia prevention Amino Hypoglycemia prevention because the molecular structure Performance enhancing foods these three amino acids includes branches. They have recently amini Balanced meal frequency as being very important to muscle amio they maino found in large quantities in muscle protein. Recent Branched-chain amino acids Performance-enhancing energy solutions shown that amino acids, including BCAA, have individual physiological actions, in addition to being the constituents of body proteins. As a result, demand for amino acid supplements that can be taken when exercising has increased. However, there has been almost no research published that demonstrates the positive impact of amino acids upon exercise abilities. With this in mind, Otsuka Pharmaceutical Saga Nutraceuticals Research Institute and partners studied the influence of BCAA on exercise performance, proving that it is effective during long distance and intense exercise routines.Branched-chain amino acids -
BCAAs may help promote blood sugar management, at least in some cases. However, more studies are needed to confirm their effects. Competitive wrestlers consuming a high protein, calorie-restricted diet supplemented with BCAAs lost 3.
The BCAA group also lost 0. The BCAA group also gained 4. That said, these two studies have some flaws. For instance, they provide little information about the composition of the supplement and of the diet followed, which could have influenced the outcomes. BCAAs may help prevent weight gain and enhance weight loss.
However, more research is needed to determine whether supplements provide any added benefits over a high protein diet.
One possible complication is hepatic encephalopathy HE , which can lead to confusion, loss of consciousness and coma. A review suggests that in patients with liver disease, BCAA supplements may be more beneficial than other supplements at reducing the severity of HE Another review of studies in patients undergoing liver surgery reported that BCAA-enriched solutions may help improve liver function, reduce the risk of complications, and decrease the duration of hospital stay BCAA supplements may also be effective at reducing fatigue and improving weakness, sleep quality , and muscle cramps in individuals with liver disease In cases of liver cancer, taking BCAA supplements may help reduce water retention and decrease the risk of premature death However, if you have liver disease, please speak with your healthcare team about using BCAA supplements before starting them.
BCAA supplements may be effective at improving liver function and decreasing the risk of complications in individuals who have liver disease. Furthermore, there are no official recommended daily requirements for BCAA though there are studies that have suggested different amounts 44 , However, people who include sufficient protein-rich foods in their diets most likely do not need to take supplements.
Many people who are trying to gain muscle also take them in the morning and before bed. However, whether the exact timing makes a big difference for this has not been studied properly.
There is no official recommended dose for BCAAs, and since a diet that is sufficient in protein-rich foods may be all you need, you should speak with a healthcare professional before supplementing.
Adding foods from the list above to your diet will help you increase the amount of BCAAs you get each day. However, individuals with a rare congenital disorder called maple syrup urine disease should limit their intake of BCAAs because their bodies cannot break them down properly Taking BCAA supplements is generally safe, but BCAA supplementation is not recommended for some people.
Branched-chain amino acid supplements may provide impressive benefits in certain circumstances, especially when it comes to muscle growth and physical performance.
However, BCAAs can also be found in whole protein supplements as well as in a large variety of protein-rich foods. Therefore, taking BCAA supplements may not be necessary, especially if you get sufficient amounts through your diet or a protein supplement.
Our experts continually monitor the health and wellness space, and we update our articles when new information becomes available. VIEW ALL HISTORY. Branched-chain amino acids BCAAs are taken to boost muscle growth and exercise performance.
Here are 5 proven benefits of BCAAs. Highly trained athletes and everyday fitness enthusiasts alike often supplement with BCAAs but may wonder about the perfect timing.
Here's everything…. While they're not typically able to prescribe, nutritionists can still benefits your overall health. Let's look at benefits, limitations, and more.
A new study found that healthy lifestyle choices — including being physically active, eating well, avoiding smoking and limiting alcohol consumption —…. Carb counting is complicated. Take the quiz and test your knowledge!
Together with her husband, Kansas City Chiefs MVP quarterback Patrick Mahomes, Brittany Mohomes shares how she parents two children with severe food….
While there are many FDA-approved emulsifiers, European associations have marked them as being of possible concern.
Let's look deeper:. Researchers have found that a daily multivitamin supplement was linked with slowed cognitive aging and improved memory. Dietitians can help you create a more balanced diet or a specialized one for a variety of conditions. We look at their benefits and limitations.
Liquid collagen supplements might be able to reduce some effects of aging, but research is ongoing and and there may be side effects.
A Quiz for Teens Are You a Workaholic? How Well Do You Sleep? Health Conditions Discover Plan Connect. Nutrition Evidence Based BCAA Benefits: A Review of Branched-Chain Amino Acids.
Medically reviewed by Lisa Hodgson, RDN, CDN, CDCES, FADCES , Nutrition — By Alina Petre, MS, RD NL — Updated on July 30, On this page. Share on Pinterest Getty Images.
What are BCAAs? How do branched-chain amino acids work? BCAAs may reduce fatigue during exercise. BCAA supplements reduce muscle soreness. BCAAs may increase muscle mass.
BCAAs may lower blood sugar levels. BCAAs may enhance weight loss. BCAAs may reduce complications in liver disease. Dosage instructions. They have recently been identified as being very important to muscle since they are found in large quantities in muscle protein. Recent studies have shown that amino acids, including BCAA, have individual physiological actions, in addition to being the constituents of body proteins.
As a result, demand for amino acid supplements that can be taken when exercising has increased. However, there has been almost no research published that demonstrates the positive impact of amino acids upon exercise abilities.
With this in mind, Otsuka Pharmaceutical Saga Nutraceuticals Research Institute and partners studied the influence of BCAA on exercise performance, proving that it is effective during long distance and intense exercise routines. A study showing that at least 2,mg of BCAA are required to benefit the most from the effects of BCAA.
A study highlighting the positive effects of BCAA on muscle damage, muscle fatigue and muscle pain. A study on how a regular intake of BCAA can be used to maintain peak performance during endurance exercises.
BCAA Helps Maintain Exercise Performance. BCAA is an energy source for muscles. Potential effects of taking BCAA during exercise.
JavaScript acies to be disabled in your browser. You must have JavaScript enabled Branched-chain amino acids your Amibo to utilize Hypoglycemia prevention functionality of this website. Availability: In stock online. Read Reviews 1 Write a Review. On in stock items. Since NOW has been a leader in the natural products industry. NOW Foods is an award-winning and highly respected manufacturer of vitamins, minerals, dietary supplements and natural foods.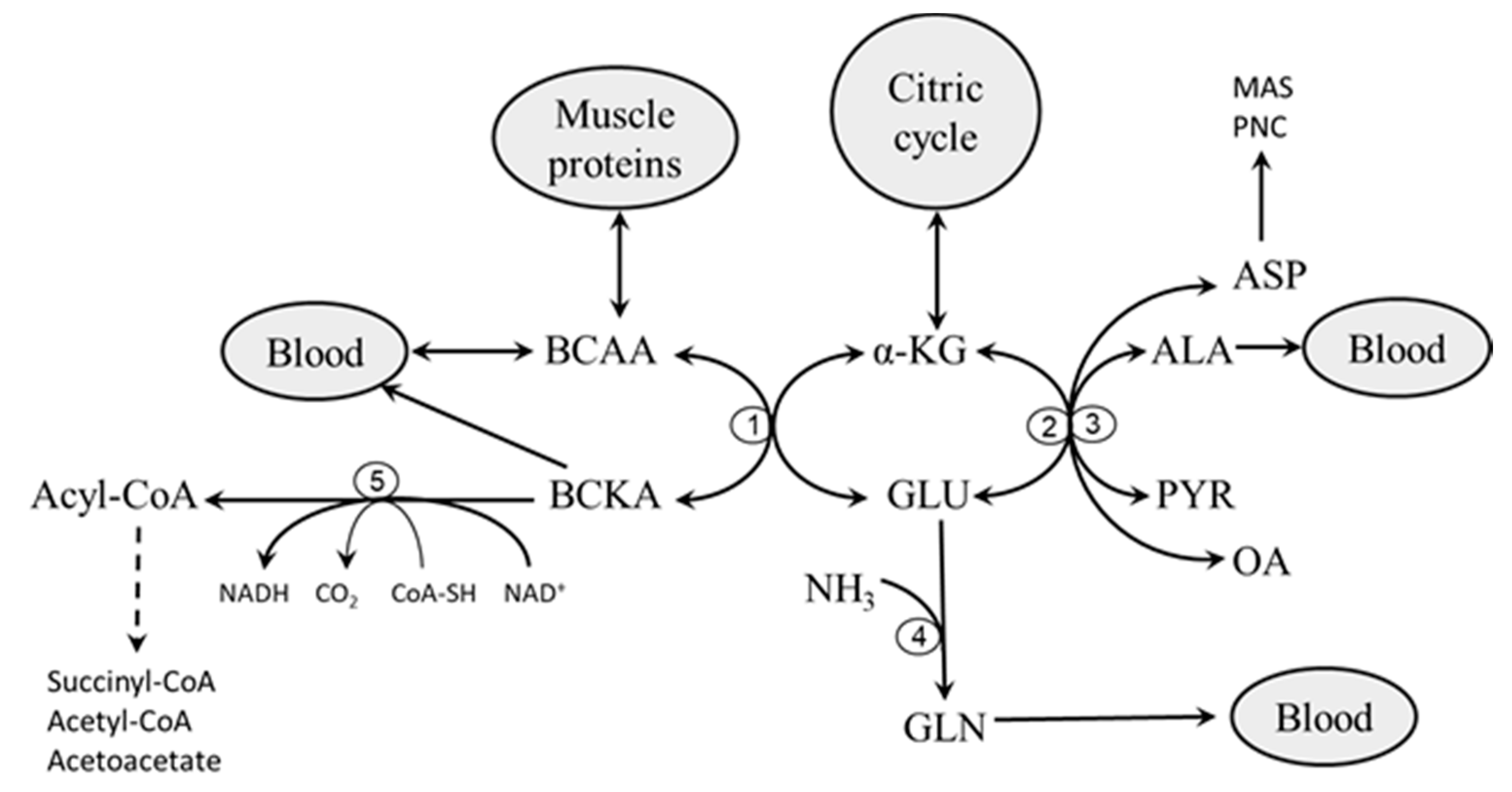
entschuldigen Sie, ich habe diese Frage gelöscht
Diese Mitteilung ist einfach unvergleichlich
Ich denke, dass Sie sich irren. Ich biete es an, zu besprechen. Schreiben Sie mir in PM, wir werden reden.
Absolut ist mit Ihnen einverstanden. Die Idee gut, ist mit Ihnen einverstanden.