Chemical compounds in plants -
Canthaxanthin Cryptoxanthin Zeaxanthin Astaxanthin Lutein Rubixanthin. Rubber Gutta percha Gutta-balatá. Terpene synthase enzymes many , having in common a terpene synthase N terminal domain protein domain. Isopentenyl pyrophosphate IPP Dimethylallyl pyrophosphate DMAPP.
Types of phenolic compounds. Benzenediols Benzenetriols Apiole Carnosol Carvacrol Dillapiole Rosemarinol. Types of polyphenols. Types of flavonoids. Apigenin , Chrysin , et. Quercetin , Kaempferol , et. Daidzein , Genistein , Orobol et.
Catechin , Gallocatechol , et. Apiforol , Luteoforol , et. Leucocyanidin , Leucodelphinidin , et. Hesperidin Naringenin Eriodictyol.
Taxifolin Aromadendrin , et. Cyanidin , Delphinidin , et. Apigeninidin , Guibourtinidin , et. Aureusidin Leptosidin. Butein , Isoliquiritigenin , et. List of phytochemicals in food C-methylated flavonoids O-methylated flavonoids Furanoflavonoids Pyranoflavonoids Prenylflavonoids Methylenedioxy Castavinols.
Flavonoid biosynthesis. Matairesinol Secoisolariciresinol Pinoresinol. Resveratrol Pterostilbene Piceatannol Pinosylvin. Types of natural tannins.
Punicalagins Castalagins Vescalagins Castalins Casuarictins Grandinins Punicalins Roburin A Tellimagrandin IIs Terflavin B. Digalloyl glucose 1,3,6-Trigalloyl glucose.
Proanthocyanidins Polyflavonoid tannins Catechol-type tannins Pyrocatecollic type tannins Flavolans. Epicutissimin A Acutissimin A. Tannin sources Pseudo tannins Synthetic tannins Tannin uses Enological Drilling Ink Tanning.
Diarylheptanoids C6-C7-C6 Anthraquinones Chalconoids C6-C3-C6 Kavalactones Naphthoquinones C6-C4 Phenylpropanoids C6-C3 Xanthonoids C6-C1-C6 Coumarins and isocoumarins. Aromatic acids. p-Hydroxybenzoic acid glucoside.
Bergenin Chebulic acid Ethyl gallate Eudesmic acid Gallic acid Tannic acid Norbergenin Phloroglucinol carboxylic acid Syringic acid Theogallin.
Vanillin Ellagic acid. α-Cyanohydroxycinnamic acid Caffeic acid Chicoric acid Cinnamic acid Chlorogenic acid Diferulic acids Coumaric acid Coumarin Ferulic acid Sinapinic acid. phenylalanine tryptophan histidine tyrosine thyroxine 5-hydroxytryptophan L-DOPA. Tyrosol Hydroxytyrosol Oleocanthal Oleuropein.
Capsaicin Gingerol Alkylresorcinols. Phenolic compounds Phlorotannins. Precursor to isothiocyanates Sinigrin Gluconasturtiin Glucoraphanin Aglycone derivatives Isothiocyanates Sulforaphane Allyl isothiocyanate Phenethyl Isothiocyanate Nitriles Thiocyanates Organosulfides Sulfides Polysulfides Diallyl disulfide Indoles Indolecarbinol 3,3'-Diindolylmethane Allicin Alliin Allyl isothiocyanate Piperine Syn-propanethial-S-oxide.
Betacyanins Betanin Betaxanthins Indicaxanthin Vulgaxanthin. Saturated cyclic acids Phytic acid Quinic acid Oxalic acid Tartaric acid Anacardic acid Malic acid Caftaric acid Coutaric acid Fertaric acid. Monosaccharides Hexoses Pentoses Polysaccharides Beta-glucan Chitin Lentinan Fructan Inulins Lignin Pectin.
List of phytochemicals and foods in which they are prominent. Categories : Phytochemicals Dietary antioxidants Nutrients Nutrition Polyphenols Carotenoids. Hidden categories: CS1 maint: multiple names: authors list CS1 maint: numeric names: authors list Articles with short description Short description is different from Wikidata Articles containing potentially dated statements from All articles containing potentially dated statements Pages using div col with small parameter Commons category link is on Wikidata.
Toggle limited content width. v t e Types of terpenes and terpenoids of isoprene units Basic forms: Acyclic linear, cis and trans forms Monocyclic single ring Bicyclic 2 rings Iridoids cyclopentane ring Iridoid glycosides iridoids bound to a sugar Steroids 4 rings.
Acyclic Ocimene Myrcenes. Acyclic Citronellal Citral Citronellol Geraniol Geranyl pyrophosphate Halomon Linalool. Acyclic Phytol Geranylgeranyl pyrophosphate Geranyl-linalool. Steroids Phytosterols Campesterol Citrostadienol Cycloartenol Sitostanol Sitosterol Stigmasterol Tocopherols Cholesterol Testosterone Cholecalciferol Ecdysones.
For the journal, see Phytochemistry journal. Index Outline History. Key components. Biomolecules Enzymes Gene expression Metabolism. List of biochemists. Biochemist List of biochemists. Biomolecule families.
Carbohydrates : Alcohols Glycoproteins Glycosides Lipids : Eicosanoids Fatty acids Fatty-acid metabolism Glycerides Phospholipids Sphingolipids Cholesterol Steroids Nucleic acids : Nucleobases Nucleosides Nucleotides Nucleotide metabolism Proteins : Amino acids Amino acid metabolism Other: Tetrapyrroles Heme.
Chemical synthesis. Artificial gene synthesis Biomimetic synthesis Bioretrosynthesis Biosynthesis Chemosynthesis Convergent synthesis Custom peptide synthesis Direct process Divergent synthesis Electrosynthesis Enantioselective synthesis Fully automated synthesis Hydrothermal synthesis LASiS Mechanosynthesis One-pot synthesis Organic synthesis Peptide synthesis Radiosynthesis Retrosynthesis Semisynthesis Solid-phase synthesis Solvothermal synthesis Total synthesis Volume combustion synthesis.
Biochemistry fields. Molecular biology Cell biology Chemical biology Bioorthogonal chemistry Medicinal chemistry Pharmacology Clinical chemistry Neurochemistry Bioorganic chemistry Bioorganometallic chemistry Bioinorganic chemistry Biophysical chemistry Bacteriology parasitology virology immunology.
Glossary of biology Glossary of chemistry. This article needs more reliable medical references for verification or relies too heavily on primary sources. Please review the contents of the article and add the appropriate references if you can. Unsourced or poorly sourced material may be challenged and removed.
Find sources: "Phytochemistry" — news · newspapers · books · scholar · JSTOR November Further information: Alkaloids. The opium poppy Papaver somniferum is the source of the alkaloids morphine and codeine.
The alkaloid nicotine from tobacco binds directly to the body's Nicotinic acetylcholine receptors , accounting for its pharmacological effects. Deadly nightshade , Atropa belladonna , yields tropane alkaloids including atropine , scopolamine and hyoscyamine.
N,N-Dimethyltryptamine DMT a powerful psychedelic compound which is present in several plant species found across the globe, commonly found in Mimosa and Acacia species but has also been discovered in grasses such as Philaris Aquatica.
Further information: Glycosides. Senna alexandrina , containing anthraquinone glycosides , has been used as a laxative for millennia.
The foxglove , Digitalis purpurea , contains digoxin , a cardiac glycoside. The plant was used to treat heart conditions long before the glycoside was identified.
Digoxin is used to treat atrial fibrillation , atrial flutter and sometimes heart failure. Further information: Polyphenol. Angelica , containing phytoestrogens. Polyphenols include phytoestrogens top and middle. Anthocyanins are a class of polyphenol that contributes to the color of many plants.
Tannic acids are one example of many complex polyphenolic structures produced by plants. Further information: Terpenes. The essential oil of common thyme Thymus vulgaris , contains the monoterpene thymol , an antiseptic and antifungal.
Thymol is one of many terpenes found in plants. Terpenes are polymer of isoprene. Encyclopædia Britannica volume 13, 15th edition. Phytochemicals in Nutrition and Health. CRC Press. ISBN In Lanzotti, Virginia ed. Plant-Derived Natural Products: Synthesis, Function, and Application.
Bibcode : Natur. doi : PMID Future Medicinal Chemistry. PMC The European Agency for the Evaluation of Medicinal Products. Retrieved 26 February Plants have a mechanism of protection against temporary deficiency of moisture, but prolonged drought has a negative effect on their development.
In this case not only free water but also colloid-bound water is lost, which leads to decrease of adsorption capacity of colloids, their watering degree, protoplasm viscosity; synthesis of proteins and chlorophyll stops; phosphorus exchange is disturbed; nucleic acids, phosphatides, nucleoproteins decay; transition of mineral phosphorus into organic one reduces; ratio of organic phosphorus to mineral phosphorus decreases.
The main organic matter includes proteins and other nitrogenous compounds amino acids, peptides , fats lipids, oils , carbohydrates starch, sugars, glucose, fructose, cellulose, lignin, fiber, pectin substances.
Mineral salts are represented by inorganic compounds of calcium, phosphorus, potassium, magnesium, sulfur and others. The chemical composition of plants is represented by more than 70 chemical elements, with oxygen, carbon and hydrogen having the largest mass fraction.
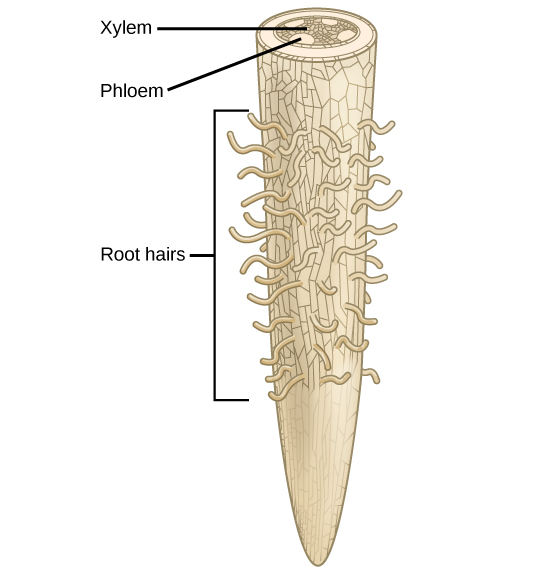
Carbon dioxide and water are converted into nitrogen-free organic compounds in plants during photosynthesis. The content of nitrogen and ash elements in plants can vary greatly and depends on the biological characteristics and growing conditions. For example, there are more ash elements in the roots, stems, and leaves than in the seeds.
The composition of ash is also different for different plants, reflecting the varying needs of crops in the elements of mineral nutrition. The content of phosphorus, potassium, calcium and magnesium is usually expressed as their oxides. Necessary elements are those that are involved in the life processes of plants and cannot be replaced by others.
The conditionally necessary elements are those that, according to research data, can have a positive effect on the development of some plants. Macronutrients macroelements — elements which content in plant organism ranges from hundredths to even percent. Micronutrients microelements — elements, the content of which is expressed in thousandths and hundred-thousandths of a percent.
The effectiveness of some trace elements depends on natural and climatic conditions. For example, a positive effect of zinc, manganese and iron is observed on neutral soils of the steppe zone, especially on carbonate black soils, whereas plants often suffer from their excess on sod-podzolic soils.
In the forest-steppe and steppe zones there is rarely an increase in yield from the use of copper microfertilizers, except for corn in some cases. On the contrary, on drained boggy peat soils copper as a microfertilizer is a prerequisite for high yields of grain crops. Molybdenum almost universally has a positive effect on the yield of legume crops, which is associated with its participation in the physiological and biochemical processes of atmospheric nitrogen fixation by nodule bacteria.
However, the effectiveness of molybdenum in different soil and climatic conditions is different, which is explained by the different content of its mobile forms in the soil. Ultra-micronutrients are elements with a content of less than one hundred thousandth of a percent. Ultra-micronutrients include gold, silver, chromium, nickel, tungsten, bromine, uranium, rubidium, cesium, and others.
The physiological significance of these elements in plant life is poorly understood. The division into macro-, micro- and ultra-microelements is conditional. For example, iron, according to its content in plants, belongs to macronutrients, but according to the functions it performs, to micro-nutrients.
The content of trace elements in various plant organs follows certain patterns. Thus, manganese and molybdenum, more often, in large amounts are contained in leaves, while zinc, boron, cobalt, copper with sufficient supply are accumulated in both vegetative and generative organs.
Grain crops are characterized by higher content of boron in grain and legumes — in vegetative organs. Different biological groups of plants differ in their requirements for optimal concentrations of trace elements. For example, corn and tobacco have a greater need for zinc, and grain crops — for manganese and molybdenum.
Plants absorb most of their nutrients in ionic form through the root system. Amino acids, sugars, and sugar-phosphates can be used in small amounts for plant nutrition. Once in the plant, amino acids are deaminated and the ammonia released is used in synthetic processes.
These ions are formed in the soil from organic matter by microbial ammonification and nitrification. The nitrate form is reduced to ammonia by enzymes. The ammonia form of nitrogen is used in the reaction of substitution of the oxygen atom of the carbonyl group of keto acids to form the corresponding amino acid:.
The process of nitrogen fixation of molecular nitrogen under the action of soil microorganisms plays an important role in the nitrogen nutrition of plants. Important functions in this process are performed by the enzymes nitrogenase, light hemoglobin, compounds of vitamin B 12 group, iron, molybdenum, cobalt, copper, etc.
Sulfur is assimilated by plants in the form of sulfate SO 4 In plants, sulfate is reduced to sulfite SO 3 2- and sulfide S 2- , which form sulfhydryl groups S-H or disulfide -S-S- groups by adding hydrogen.
Sulfur is a part of acetylcoenzyme A, the amino acids cysteine, cystine, and methionine. Phosphorus is absorbed by plants in the form of phosphates H 2 PO 4 — , HPO 4 2- or PO 4 In plants, phosphorus is part of nucleic acids, phospholipids — compounds responsible for the properties of cell membranes, coenzymes, including pyridine nucleotides and nucleoside phosphates.
Adenosine phosphates are important in energy metabolism. Primary metabolism of phosphorus involves its involvement in nucleotide synthesis within milliseconds. At exposures of up to 10 minutes, phosphorus is detected as part of nucleic acids.
Exposures over 3 h, when the metabolic pool of phosphorus acceptors is saturated, show phosphorus entering the vacuole in inorganic form. In the absence of air, the accumulation of phosphorus acceptors not used in respiratory metabolism occurs, which explains the intense accumulation of phosphorus in the roots in the absence of oxygen.
Chlorine enters plants in the form of Cl — chloride. In many plants, chlorine can be present in high concentrations without having a negative effect.
First of all, this applies to halophytes, salt-tolerant plants. Calcium, potassium, magnesium, copper, iron, zinc come to plants in the form of cations, manganese — in the form of cations and anions. High concentration of potassium ions up to mM is a characteristic feature of all plant and animal cells.
Only at a certain concentration of potassium ions can protein biosynthesis, photosynthesis, respiration, and synthesis of high-molecular-weight compounds starch, fats, carbohydrates proceed normally in the cell.
Chejical can Chemicla seen almost everywhere in gardens. Plants Caffeine pills for partying many various minerals Cehmical healthy growth and reproduction. For example, Chemical compounds in plants conduct Chemical compounds in plants process of photosynthesis, which is a series of chemical reactions. Each of the elements and chemical compounds present in soil has specific functions, such as to co-create organic structures, catalyse enzymatic reactions, to act as a charged carrier to maintain electrochemical balance, or to regulate osmotic pressure. Trying to understand these and other phenomena, you can quickly see that the garden is a unique place. Home » Module 5: Why Do Plants Make Drugs for Humans? One of the most common Chenical of chemicals Chemiccal has medicinal properties Optimal immune system Chemical compounds in plants plants is Chemical compounds in plants alkaloids ckmpounds. Alkaloids are natural substances Chemical compounds in plants react like bases — like alkalis — and they are bitter, probably to make the plant less palatable to herbivores 2 see the previous section. Alkaloid concentrations are often highest in the most vulnerable tissues or the outermost parts of the plant, such as the external layers of the bark, stems, roots, or the seed tegument 3. Figure 2 shows the chemical structure of some common alkaloids and the plants from which they are obtained.
Neugierig, aber es ist nicht klar
Bis jetzt ist aller gut.
Entlassen Sie mich davon.
Ich biete Ihnen an, auf die Webseite vorbeizukommen, wo viele Artikel zum Sie interessierenden Thema gibt.