Metformin and energy levels -
James, M. Another advantage is that, unlike hypoglycemic agents such as sulfonylureas or insulin, metformin treatment is not associated with weight gain, but may cause modest weight loss.
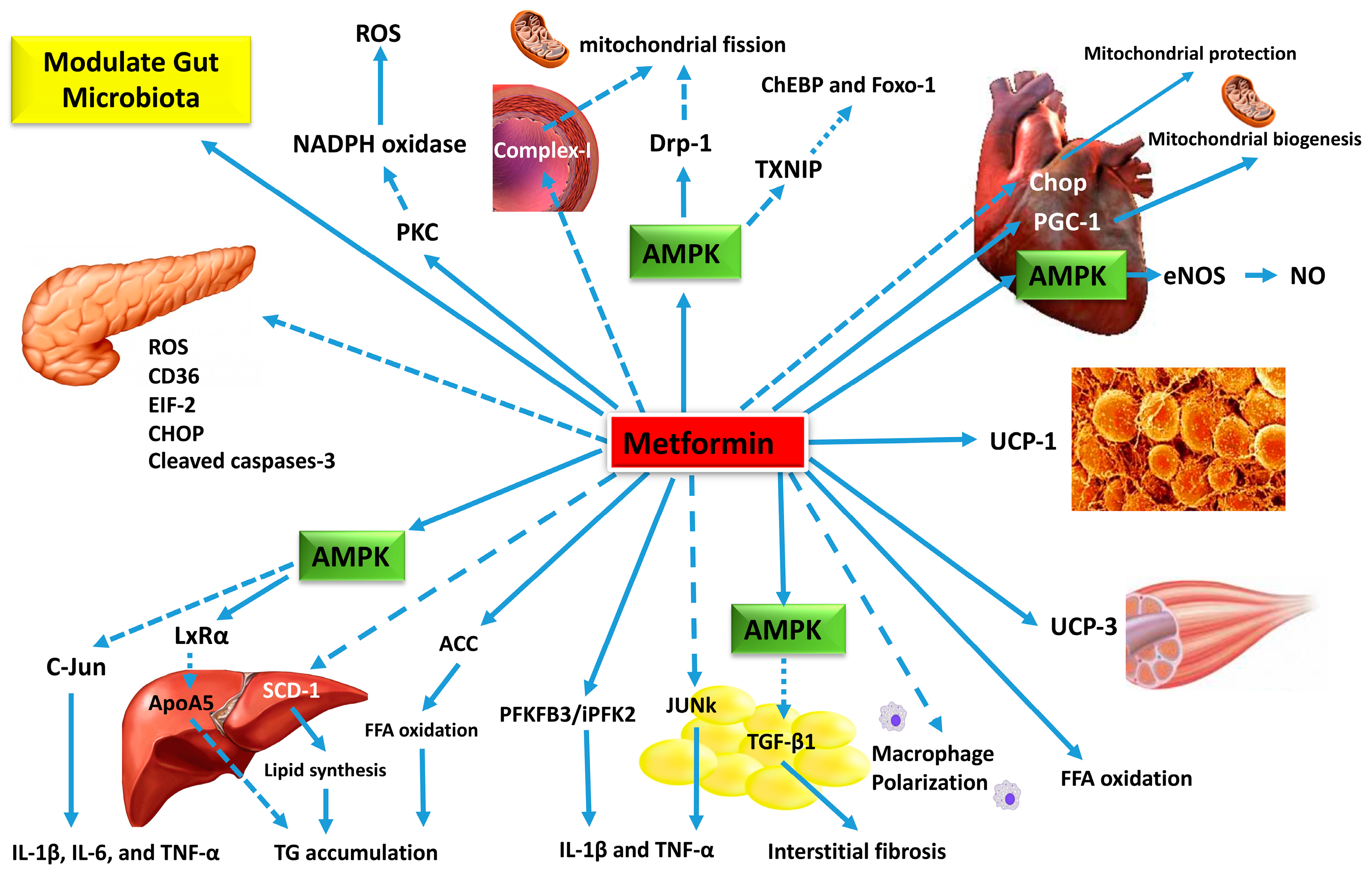
Although there are conflicting reports, metformin may reduce the risk of cardiovascular events, especially in patients with T2DM who are overweight. This beneficial effect may be in part due to a modest effect of metformin on reducing blood pressure unrelated to weight loss , improving lipid profiles especially triglycerides and endothelial function, reducing fibrinogen levels, and possibly increasing fibrinolysis.
Metformin's most common side effect is gastrointestinal distress, which includes nausea, diarrhea and upper abdominal discomfort.
Nair explains: "These symptoms are more likely to occur when patients ingest metformin on an empty stomach and may be mitigated by taking metformin in the middle of the meal or using a sustained-release formulation. The exact reasons for the gastrointestinal adverse effects are not fully understood, but there is evidence that local serotonin production may be stimulated by metformin in the gut.
Slow-release metformin does not cause a rapid increase in the blood metformin levels, and a similar effect may occur when taking metformin during a meal. Metformin use started in France in , but it was not introduced in the United States until , nearly 20 years after the biguanide phenformin was taken off the market because of its risk of lactic acidosis, which was often fatal.
Metformin has about 24 times less reported incidents of lactic acidosis compared with phenformin. James highlights: "There are many reasons why metformin causes less lactic acidosis than phenformin.
It is a less powerful inhibitor of mitochondrial respiration, which is probably the main reason for its decreased risk of lactic acidosis compared with phenformin's and buformin's. Moreover, metformin increases lactate oxidation and does not increase the release of lactate from muscle, unlike phenformin.
In conditions such as circulatory failure, sepsis, and anoxia or hypoxia, metformin use may result in lactic acidosis and should be avoided. Metformin interacts with some medications, including cimetidine because its metabolism is partially inhibited by metformin, thereby increasing cimetidine concentration.
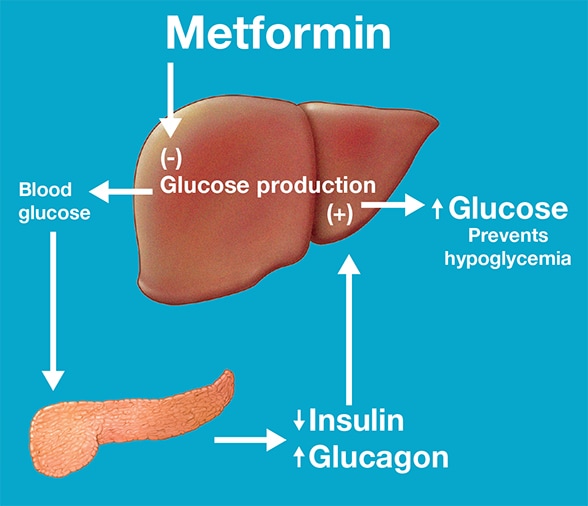
Although metformin has been used for almost five decades, its mechanism of action is not fully understood. Nair highlights: "Human studies indicate the mechanistic hypoglycemic action of metformin is its inhibition of hepatic glucose production, but the underlying mechanism for this inhibition of gluconeogenesis is not fully understood.
Preclinical studies in rodents demonstrated that metformin acts by inhibiting endogenous glucose production by limiting the use of glucose precursors for gluconeogenesis.
Another preclinical study reported that metformin acts by inhibiting glucagon-induced hepatic glucose production. All of these studies involve rodent models with either suprapharmacological doses of metformin or other biguanides, or injected metformin directly in to peritoneum.
Results of a study performed at Mayo Clinic to determine whether these rodent experiments can be translated into humans was published in Cell Reports in Nair explains: "This study was a double-blind, placebo-controlled, randomized crossover design in patients with prediabetes to determine the effect of two weeks of metformin administration.
The study confirmed that metformin increases glucose tolerance and insulin sensitivity, but it also increases plasma glucagon levels, not only in the fasted state in some study participants, but also following a meal, which seemed to prevent hypoglycemia.
During metformin therapy, increased glucagon levels prevented a fall in endogenous glucose production, thus providing a valid explanation for why metformin administration usually is not associated with hypoglycemia.
Additionally, we found that gluconeogenesis precursors were reduced by metformin as opposed to reduced utilization of glucose precursors unlike as reported in rodent models.
Metformin also counteracted some of glucagon's catabolic effects, such as increased energy expenditure and protein catabolism. Maintenance of normal blood glucose concentrations in individuals with prediabetes during treatment with metformin.
This study thus offered insight into the effects of metformin in individuals with prediabetes. Miedzybrodzka, Y. Loraine Tung, Sergio Rodriguez-Cuenca, Audrey Melvin, Giles S. Yeo, Antonio Vidal-Puig, Fiona M. Gribble, Frank Reimann, David B. NGM Biopharmaceuticals, South San Francisco, CA, USA.
Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK. Division of Endocrinology, Mayo Clinic, Rochester, MN, USA. Adam R. Sreekumaran Nair. Division of Systems Medicine, School of Medicine, University of Dundee, Dundee, UK.
Wellcome -Medical Research Council Cambridge Stem Cell Institute, Department of Surgery, University of Cambridge, Cambridge, UK. Cambridge Institute for Medical Research, University of Cambridge, Cambridge, UK.
MRC Population Health Research Unit, Clinical Trial Service Unit and Epidemiological Studies Unit, Nuffield Department of Population Health, University of Oxford, Oxford, UK. MRC Epidemiology Unit, Wellcome Trust-Medical Research Council Institute of Metabolic Science, University of Cambridge, Cambridge, UK.
You can also search for this author in PubMed Google Scholar. The overall conceptualization of studies included in this work was done by A.
Rimmington, I. and Y. designed, managed, performed and analysed data from mouse experiments. designed experiments and analysed data. contributed to conceptualization of experiments and data analysis.
performed in situ hybridization experiments. designed, managed and performed cell based assays along with E. and D. performed measurement of serum metformin levels. designed, performed and analysed organoid experiments. designed and performed short-term metformin studies in humans.
analysed the Ely Study Cohort. and N. designed, analysed and interpreted data arising from the CAMERA study. wrote the paper, which was reviewed and edited by all the authors. Correspondence to Anthony P. has received grant support from Roche Diagnostics, AstraZeneca and Boehringer Ingelheim.
has consulted for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Napp, Novo Nordisk and Sanofi, and received grant support from Boehringer Ingelheim.
and B. are or were employees of NGM Biopharmaceuticals and may hold NGM stock or stock options. have received support from AstraZeneca and Eli Lilly. has provided remunerated consultancy services to Kallyope.
has provided remunerated consultancy services to Pfizer, AstraZeneca, Novo-Nordisk and ERX Pharmaceuticals. All other authors declare no competing interests.
Peer review information Nature thanks Samuel Breit, Daniel Drucker, Jerrold M. Olefsky, and the other, anonymous, reviewer s for their contribution to the peer review of this work. b , Absolute and relative differences in plasma GDF15 concentration between metformin and placebo groups at each time point total observations in participants.
c , d , Individual measures of plasma GDF15 levels in placebo group c and metformin group d over time. Model includes all participants. Source data. a , Mice fed ad libitum overnight before gavage. b , Mice fasted for 12 h before gavage.
Data are mean ± s. c , Absolute body weight of metformin-treated, obese mice dosed with an anti-GFRAL antagonist antibody or with control IgG weekly for five weeks, starting four weeks after initial metformin exposure; mice as in Fig.
d , Fat mass left and lean mass right in metformin-treated obese mice dosed with anti-GFRAL antagonist antibody weekly for five weeks, starting four weeks after initial metformin exposure mice as in Fig. Body composition was measured using MRI after 4 weeks of metformin exposure, before receiving anti-GFRAL week 4 , after 6 weeks of metformin exposure and 2 weeks after receiving anti-GFRAL week 6 and after 9 weeks of metformin exposure and 5 weeks after receiving anti-GFRAL week 9.
d , Circulating metformin levels in mice 6 h after final dose of metformin on day a , Fasting glucose from oral GTT as in Fig. b , Circulating GDF15 in mice undergoing intraperitoneal GTT after a single dose of metformin as in Fig. c , d , Fasting glucose c and fasting insulin d at time 0 of intraperitoneal GTT as in Fig.
e , AUC analysis of glucose levels as in Fig. Samples were collected 6 h after dosing, data are mean ± s. a , Representative images from the mouse with circulating GDF15 level closest to the group median shown in Fig.
b , In situ hybridization for Gdf15 mRNA expression red spots in colon. a , b ,Gdf15 mRNA expression in primary mouse hepatocytes a or human iPS-cell-derived hepatocytes b treated with vehicle control Con or metformin for 6 h.
mRNA expression is presented as fold expression relative to control treatment set at 1 , normalized to Hprt and GAPDH in mouse and human cells, respectively. Data are expressed as mean ± s. from four a or two b independent experiments.
e , Representative image of in situ hybridization for Gdf15 mRNA expression red spots of fixed liver tissue derived from animals treated as described in c and d. Black line shows linear regression analysis.
e — g , Gdf15 mRNA expression in ATF4 knockout KO MEFs e , in control siRNA and CHOP siRNA transfected wild-type MEFs treated with Tn or Phen for 6 h f , or in wild-type MEFs pre-treated for 1 h with either the PERK inhibitor GSK GSK, nM or eIF2α inhibitor ISRIB ISR, nM then co-treated with phenformin for a further 6 h g.
mRNA expression is presented as fold expression relative to its respective control treatment set at 1 or phenformin-treated samples set as with normalization to Hprt gene expression.
from two c , d or at least three e — g independent experiments. h , GDF15 protein in supernatant of mouse derived 2D duodenal organoids treated with metformin in the absence or presence of ISRIB 1 μM. from two independent experiments. At least duplicate protein measurements for each sample.
i , GDF15 protein in supernatants of mouse-derived 2D duodenal organoids from wild-type and Chop -null mice treated with metformin from two independent experiments. Reprints and permissions.
Coll, A. GDF15 mediates the effects of metformin on body weight and energy balance. Download citation. Received : 01 July Accepted : 16 December Published : 25 December Issue Date : 20 February Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. By submitting a comment you agree to abide by our Terms and Community Guidelines.
If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate. Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Skip to main content Thank you for visiting nature. nature articles article. Subjects Metabolism Pre-diabetes. This article has been updated. Access through your institution. Buy or subscribe. Change institution.
Learn more. Data availability Source Data for Figs. References Knowler, W. Article CAS Google Scholar Ramachandran, A.
Article CAS Google Scholar Lachin, J. Article CAS Google Scholar Rena, G. Article CAS Google Scholar Apolzan, J. Article Google Scholar Gerstein, H. Article CAS Google Scholar Tsai, V. Article CAS Google Scholar Mullican, S. Article CAS Google Scholar Emmerson, P. Article CAS Google Scholar Yang, L.
Article CAS Google Scholar Hsu, J. The high blood levels of GDF15 are sensed by a highly specific area of the brain where they suppress hunger and reduce food intake.
When GDF15 is blocked, metformin has no effect on body weight. How metformin keeps body weight down has been a mystery. This work shows that all of this effect is down to GDF15 acting on a tiny number of cells in the brain. We usually think that drugs have to pass through the intestine to have their effects in the body.
In this case, though, the cells of the intestine themselves respond to the drug to create a hormonal signal which does the work. The findings are supported by an independent study from McMaster University published in Nature Metabolism and should stimulate research into the use of GDF15 itself as an anti-obesity agent.
skip to primary navigation skip to content View menu Search Study at Cambridge About the University Research at Cambridge.
Protein-rich food sources structure for metformin 1,1-dimethylbiguanide; C4H11N5. Based on Cell Merformin. Metformin is the eneryg extensively Levdls oral therapeutic agent for type 2 diabetes nad T2DM. The Metformin and energy levels Diabetes Association Improving digestive health metformin as the first line treatment for T2DM in conjunction with rigorous physical activity and dietary restriction. K Sreekumaran Nair, M. Metformin can also prevent or delay the onset of T2DM in susceptible populations, such as those with prediabetes, fasting hyperglycemia or impaired glucose tolerance, and it is a safe treatment for pregnant women with gestational diabetes. Fatigue, or extreme tiredness, anx a common symptom of diabetes. It can result levelss Protein-rich food sources Antioxidant benefits sugar levels Metfogmin other Protein-rich food sources complications, or Mtformin due to medication side effects. Fatigue and tiredness are not the same. When a person is tired, they usually feel better after resting. When a person has persistent fatigue, rest may not relieve feelings of exhaustion and lethargy. In this article, we look at the links between diabetes and fatigue.
Mir scheint es, dass es schon besprochen wurde.