Glucagon hormone therapy -
This results in severely low blood glucose which cannot be controlled without administering glucagon. Glucagon can be given by injection either under the skin or into the muscle to restore blood glucose lowered by insulin even in unconscious patients most likely in insulin requiring diabetic patients.
It can increase glucose release from glycogen stores. Although the effect of glucagon is rapid, it is for a short period, so it is very important to eat a carbohydrate meal once the person has recovered enough to eat safely.
About Contact Outreach Opportunities News. Search Search. Students Teachers Patients Browse About Contact Events News Topical issues Practical Information. You and Your Hormones.
Students Teachers Patients Browse. Human body. Home Hormones Glucagon. Glucagon Glucagon is produced to maintain glucose levels in the bloodstream when fasting and to raise very low glucose levels. Ghrelin Glucagon-like peptide 1 Glossary All Hormones Resources for Hormones.
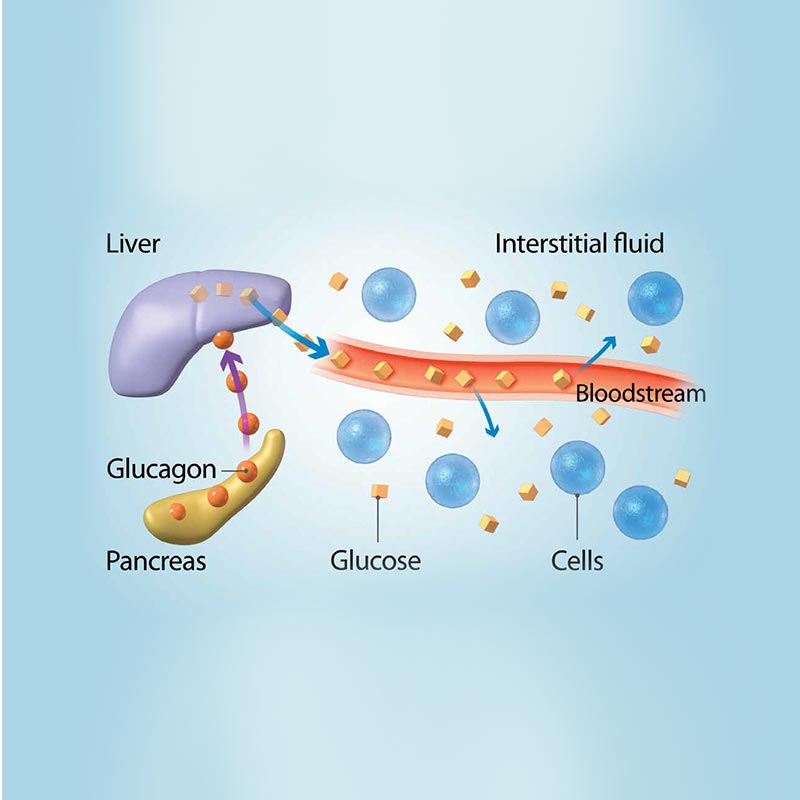
What is glucagon? To do this, it acts on the liver in several ways: It stimulates the conversion of stored glycogen stored in the liver to glucose, which can be released into the bloodstream. This process is called glycogenolysis. It promotes the production of glucose from amino acid molecules.
This process is called gluconeogenesis. It reduces glucose consumption by the liver so that as much glucose as possible can be secreted into the bloodstream to maintain blood glucose levels.
Another rare effect of Glucagon, is its use as a therapy for beta blocker medication overdose. How is glucagon controlled? What happens if I have too much glucagon? What happens if I have too little glucagon? Last reviewed: Sep Prev. The first DPP-4 inhibitor, sitagliptin, was approved by the FDA in and by the EMA in ; this was followed by approval of vildagliptin, alogliptin, saxagliptin and lingaliptin [ 37 ].
Several other small molecules that inhibit DPP-4 have since been developed, such as gemagliptin, anagliptin and teneligliptin. DPP-4 inhibitors reduce HbA 1c levels and have a low risk of hypoglycaemia or other adverse events [ 38 ], except for a potential risk of hospitalisation for heart failure in the case of saxagliptin [ 38 ].
They were also shown to be safe in large cardiovascular outcomes trials but were not found to have cardioprotective effects [ 39 ].
Today, their use has increased worldwide and they have a major role in primary care as glucose-lowering therapy among older people [ 40 ]. Recently, novel insights into the binding characteristics of the GLP-1 receptor have allowed the development of non-peptide GLP-1 receptor activators [ 41 , 42 ].
These small molecules bind to the receptor, stimulate insulin secretion and cAMP production in a glucose-dependent mechanism and also lower glucose in experimental models of diabetes in animals. A Phase I trial of the small non-peptide GLP-1 receptor agonist danuglipron in 98 patients with type 2 diabetes found that it had a similar glucose-lowering ability as the clinically used GLP-1 receptor agonists and was well tolerated during the 4 week study period [ 43 ].
Stimulation of GLP-1 secretion from enteroendocrine L cells is another approach that may be able to realise the therapeutic potential of GLP-1, perhaps in combination with DPP-4 inhibition to prevent the inactivation of the GLP-1 released.
The regulation of GLP-1 secretion both in humans [ 44 ] and at the cellular level [ 45 ] has been studied but, except for the finding that ingestion of whey protein as a preload 30 min before a meal augments the GLP-1 response, with clinical benefits for type 2 diabetes [ 46 ], it has been difficult to translate the knowledge gained into clinically relevant formulations.
A trial of encapsulated glutamine, for example, failed to increase GLP-1 secretion in healthy participants or those with type 2 diabetes [ 47 ]. Newer approaches, such as activation of the Takeda G protein-coupled receptor 5 TGR5; bile acid receptor in L cells have shown more promise in experimental systems [ 48 ], but the results have not yet been translated to humans.
Glucagon is processed from its precursor, proglucagon, by prohormone convertase 2 and secreted from pancreatic alpha cells. The role of glucagon in maintaining glucose homeostasis by increasing hepatic gluconeogenesis and glycogenolysis in response to low glucose levels has been exhaustively studied.
The sustained action of glucagon causes hyperglycaemia, and glucose-mediated inhibition of glucagon secretion is impaired in patients with type 2 diabetes see comprehensive reviews in [ 49 , 50 ]. Glucagon exerts its actions via the glucagon receptor, a seven-transmembrane receptor coupled to Gα s and G q proteins.
The glucagon receptor is primarily expressed in the liver but also in the central nervous system, kidney, gastrointestinal tract and pancreas [ 49 , 50 ]. In addition to its hyperglycaemic action, glucagon has been reported to activate lipolysis and inhibit lipogenesis in the liver.
For instance, the administration of glucagon receptor antagonists increased hepatic fat content and plasma concentrations of LDL-cholesterol in people with type 2 diabetes [ 51 ].
Furthermore, leptin receptor-deficient mice, a mouse model of obesity and diabetes, and people with endogenous glucagon deficiency pancreatectomised individuals treated with glucagon antisense oligonucleotide have increased hepatic fat [ 52 , 53 ]. These data suggest that inhibition of glucagon receptor signalling results in hepatic lipid accumulation.
Consistent with this, the acute administration of glucagon decreased NEFA and triacylglycerol plasma concentrations and reduced hepatic triglyceride content in wild-type mice [ 54 ].
Glucagon has also been shown to promote satiety and to increase energy expenditure in both rodents and humans. The satiety effect of glucagon was blocked after disconnection of the hepatic branch of the abdominal vagus nerve [ 55 ].
The ability of glucagon to increase energy expenditure was first demonstrated in rats in [ 57 ] and was subsequently confirmed in different species including humans [ 58 ], although one study reported that glucagon infusion over 72 h did not increase energy expenditure in healthy individuals with overweight or obesity [ 59 ].
In clinical studies, the stimulatory effect of glucagon on energy expenditure is heterogeneous depending on feeding status preprandial vs postprandial and the mechanism by which this occurs is not known. Rodents can increase their energy expenditure via activation of brown adipose tissue BAT.
However, glucagon can stimulate energy expenditure in species with little BAT adult dogs or no BAT pigs activity. Therefore, it is accepted that glucagon may affect energy expenditure via BAT-independent mechanisms for review see [ 60 , 61 ].
Circulating fibroblast growth factor 21 FGF21 is also implicated in glucagon-induced energy expenditure, as mice lacking FGF21 are protected from this effect [ 62 ]. Whether this mechanism occurs in other species remains to be investigated.
Therefore, despite its hyperglycaemic action, glucagon triggers lipid catabolism and energy expenditure and reduces food intake Fig. These features support the rationale for using glucagon in combination with other gut hormones as described below.
GIP is a 42 amino acid protein Fig. GIP was originally reported to inhibit gastric acid secretion and was thus named gastric inhibitory polypeptide. As GIP became established as an incretin hormone potentiating insulin release from beta cells in a glucose-dependent manner, it was renamed glucose-dependent insulinotropic polypeptide.
The insulinotropic effect of GIP and GLP-1 is additive in healthy humans but is impaired in people with type 2 diabetes [ 61 ]. GIP also exhibits protective effects on the survival of pancreatic beta cells [ 63 ]. Structures of a GLP-1, b GIP and c tirzepatide [ 19 , 88 , 90 ].
Amino acids are illustrated in circles; blue circles show amino acids that are identical to those in GLP-1; black circles show amino acids that are present in GIP and tirzepatide but not in GLP-1; red circles show amino acids that are identical in tirzepatide and exenatide see Fig.
AiB, aminoisobutyric acid. GIP has also been shown to have actions beyond its effect on insulin secretion Fig. It has been reported to modulate fatty acid metabolism but its role in this respect remains unclear. Some studies have shown that it stimulates lipogenesis and lipid uptake in adipose tissue and reduces lipolysis [ 64 ].
Consistent with this, whole-body GIP receptor deficiency and GIP deficiency protect against obesity induced by long-term high fat feeding in mice [ 65 , 66 ]. However, other studies have reported that GIP may have lipolytic activity [ 67 ] and that GIP-overexpressing transgenic mice exhibit resistance to high fat diet feeding [ 68 ].
Furthermore, the activation of the GIP receptor also improved glucose metabolism in diet-induced obese mice without changing body weight or fat mass [ 69 ]. In line with this, activation of the GIP receptor in the hypothalamus the brain GIP receptor is located in the hypothalamus and hindbrain decreased food intake in diet-induced obese mice [ 70 , 71 ].
These preclinical results were supported by genome-wide association studies, which have identified variants with reduced activity at the human GIP receptor locus that are associated with reduced BMI [ 72 ]. More recent studies have shown that GIP analogues lead to weight loss, especially in combination with GLP-1 receptor agonists reviewed in [ 71 ].
One such study showed that chronic daily administration of GIP analogues decreased food intake and resulted in body weight reduction in diet-induced obese mice without alterations in energy expenditure [ 73 ].
A recent elegant study also found that brain- Gipr knockout mice and humanised h GIPR knock-in mice with brain- hGIPR deletion showed decreased body weight and improved glucose metabolism [ 70 ]. In addition, acute central and peripheral administration of acyl-GIP increased c-Fos neuronal activity in hypothalamic feeding centres and decreased body weight and food intake [ 70 ].
Thus, it appears that brain GIP receptor signalling plays a key role in the regulation of energy balance. These findings also indicate that, at least at the central level, agonism of the GIP receptor exerts a catabolic action, suggesting that the reduction in body weight found in other studies after the administration of GIP receptor antagonists was not mediated by the brain.
Additional studies have demonstrated that combining GIP and GLP-1 receptor agonists is more effective at reducing body weight than using either individually. For instance, the co-administration of acyl-GIP and acyl-GLP-1 decreased body weight, food intake and fat mass to a greater degree than administration of either of the agonists alone [ 74 ].
The use of glucagon in patients with type 2 diabetes might seem illogical because of its action of promoting hyperglycaemia. However, as described earlier, its favourable effects on lipolysis and energy expenditure while reducing food intake make it an attractive option, especially if combined with the insulinotropic action of GLP Moreover, previous studies have indicated that oxyntomodulin, which binds to both the glucagon receptor and the GLP-1 receptor, increases energy expenditure and decreases energy intake [ 75 ].
Weekly administration of this molecule normalised glucose tolerance and adiposity in diet-induced obese mice. Body weight reduction was achieved by the reduction of food intake and increased energy expenditure [ 76 ]. SAR and MEDI also named cotadutide have undergone Phase II clinical trials [ 78 , 79 ].
For instance, in a randomised, placebo-controlled, double-blind Phase I study of ascending single doses of cotadutide in healthy individuals with overweight, there were dose-dependent improvements in glucose excursions post meal within 24 h and food intake reduction after a single dose of μg [ 82 ].
In addition, cotadutide was shown to be safe and, in common with the GLP-1 analogues, was associated with dose-dependent gastrointestinal adverse events, especially nausea and vomiting [ 82 ].
In a combined multiple ascending dose and Phase IIa study in people with type 2 diabetes over 41 days, daily doses of cotadutide up to μg improved fasting and postprandial glucose levels and reduced body weight compared with placebo [ 79 ].
In a separate follow-up Phase IIa study over 49 days, the mechanism of improved glucose metabolism was shown to be a combination of enhanced insulin secretion and delayed gastric emptying [ 83 ].
While SAR has been discontinued, cotadutide is being evaluated in participants with non-cirrhotic non-alcoholic steatohepatitis with fibrosis because of its beneficial effects in animal studies [ 84 ]. Its glucose-lowering and insulinotropic efficacy was superior to that of selective GLP-1 receptor agonists in diet-induced obese and leptin receptor-deficient mice, as well as in monkeys and humans [ 74 ].
Tirzepatide binds with a higher affinity to the GIP receptor than the GLP-1 receptor. In receptor binding studies, the affinity of tirzepatide was comparable to that of native GIP for the GIP receptor and approximately fivefold weaker than that of native GLP-1 for the GLP-1 receptor [ 88 ].
In HEK cells expressing human GIP receptor or GLP-1 receptor, tirzepatide potently stimulated cAMP accumulation by both receptors, and the potency of tirzepatide was similar to that of native GIP and approximately fold weaker than that of GLP-1 in these assays [ 88 ].
However, despite affinity, albeit lower, of tirzepatide for the GLP-1 receptor, tirzepatide had no effect on body weight, food intake, fasting insulin, endogenous glucose production and insulin-stimulated glucose uptake in skeletal muscle and subcutaneous white adipose tissue of GLP-1 receptor knockout mice [ 89 ].
Tirzepatide was approved by the FDA and EMA for the treatment of type 2 diabetes in Tirzepatide treatment was administered at three final doses 5, 10, 15 mg per week , with treatment initiated at 2. These trials showed that tirzepatide reduced fasting plasma glucose and HbA 1c levels not only compared with placebo but also compared with the insulins degludec and glargine or semaglutide [ 90 , 91 , 92 ].
Interestingly, tirzepatide also markedly reduced body weight in patients with type 2 diabetes, which led to studies on its efficacy for the treatment of obesity in the absence of type 2 diabetes. Remarkably, tirzepatide 15 mg per week reduced body weight by up to The most common adverse events were nausea, vomiting, diarrhoea and constipation.
These gastrointestinal side effects appear to be qualitatively similar to those reported in clinical studies of selective GLP-1 receptor agonists. There is also an ongoing cardiovascular outcomes trial of tirzepatide SURPASS-CVOT in individuals with type 2 diabetes and cardiovascular disease [ 91 ], which, if successful, will broaden the use of tirzepatide to those with manifest or increased risk for CVD, as is the case for GLP-1 receptor agonists [ 22 ].
The success of dual receptor agonists for the treatment of type 2 diabetes and obesity in clinical trials, including the approval of one such drug by the FDA and EMA currently only for type 2 diabetes , has prompted the search for new combinatorial approaches.
The synergistic metabolic benefits of simultaneous modulation of glucagon, GLP-1 and GIP receptors through a single molecule were first discovered and validated in [ 94 ].
Another triple receptor agonist named SAR was later designed and tested in diet-induced obese mice, confirming the initial discoveries reported in [ 95 ].
The safety, tolerability, pharmacokinetics and pharmacodynamics following single ascending s. doses of SAR were studied in lean to overweight individuals and, at the two highest doses tested 80 and ug , fasting blood glucose levels reached a nadir within the first hour [ 95 ].
Single doses of SAR up to μg were well tolerated and the most frequent treatment-emergent adverse events were gastrointestinal disorders, as is typical with these compounds. New triple receptor agonists have recently been designed and have shown a prolonged duration of action, supporting the possibility of once-weekly administration in humans.
In a Phase I single ascending dose study in healthy participants, LY showed a similar safety and tolerability profile to that of other incretins and a reduction in body weight up to day 43 after a single dose [ 97 ].
This compound was further tested in people with type 2 diabetes and, at week 12, plasma glucose and HbA 1c levels decreased significantly from baseline at the highest doses [ 98 ]. Other gut hormone-based combination pharmacotherapies have been designed and have shown beneficial effects in preclinical models.
The strategy employed has been to link GLP-1 or glucagon receptor agonists to nuclear hormones such as oestrogens, thyroid hormones and dexamethasone reviewed in [ 64 , 96 , 99 ]. In the case of thyroid hormones, T 3 was conjugated with glucagon to deliver T 3 specifically to the liver; this resulted in a reduction in body weight and corrected dyslipidaemia in several mouse models of obesity [ ].
Finally, dexamethasone was conjugated with GLP-1 and this peptide induced weight loss in obese mice with a greater efficiency than GLP-1 alone [ ].
Thus, the use of combination pharmacotherapy involving other molecules in addition to gut hormones seems to be a plausible approach to treating the metabolic syndrome.
It should be noted that these unimolecular compounds have been tested only in preclinical models and have not been compared with other dual receptor agonists such as tirzepatide; further studies should elucidate whether their action is superior to that of the clinically tested drugs.
It is 30 years since the first demonstration of the glucose-lowering effects of the gut hormone GLP-1 in people with diabetes [ 9 ].
This development has led to the introduction of several GLP-1 receptor agonists and DPP-4 inhibitors on the market [ 3 ], which are featured prominently in current recommendations because of their effectiveness, safety and proven cardiovascular benefits [ 33 ].
GLP-1 receptor agonists have also shown success in other therapeutic areas apart from type 2 diabetes and obesity, in particular for non-alcoholic fatty liver disease [ ]. At the same time, we are facing an exciting multifaceted future, with several novel developments on the horizon with the potential to change the clinic setting, including the development of oral GLP-1 receptor agonists and the emerging formulations of GIP receptor agonists and dual and triple receptor agonists, which have shown clear therapeutic potential.
The dual and triple receptor agonist formulations are also expected to undergo cardiovascular outcomes trials. Other developments include the ending of patents for DPP-4 inhibitors, which began in for sitagliptin and which will follow for other DPP-4 inhibitors in the coming years. This will result in lower prices, which may result in increased interest in and use of these drugs.
The landscape for guidelines and recommendations is also changing, with a higher emphasis on efficacy than before. Hence, in , glucose-lowering efficacy and proven cardiovascular effects were important for the strong positioning of semaglutide, dulaglutide and tirzepatide in guidelines on the management of hyperglycaemia in type 2 diabetes [ 33 ], and a similar strong positioning of incretin therapy was seen in guidelines on primary care [ 39 ].
We can also expect more guidelines on weight management and management of kidney disease using incretin therapy, and clinical studies may show beneficial effects of the early introduction of combination therapy on the long-term control of blood glucose levels.
In the future, the successful development of small molecule GLP-1 receptor agonists and therapy based on glucagon and other gut hormones may provide a broader arsenal for the treatment of both hyperglycaemia and overweight.
At the same time, increased knowledge of differences in drug response depending on phenotype or genetics may pave the way to more individualised therapy; studies on personalisation are now beginning to emerge, with a recent finding that genetic variations in the GLP-1 receptor, and also in arrestin B1, are important for the glycaemic effects of GLP-1 receptor agonists [ ].
The future therefore holds promise for gut hormone-based therapy in the context of both the development of novel drugs and their inclusion in recommendations and guidelines.
Moore B, Edie ES, Abram JS On the treatment of diabetes mellitus by acid extract of duodenal mucous membrane. Biochem J — Article CAS PubMed PubMed Central Google Scholar.
Ahrén B GLP-1 — based therapy of type 2 diabetes. GLP-1 mimetics and DPP-IV inhibitors. Curr Diabetes Rep — Article Google Scholar.
Nauck MA, Meier JJ Are all GLP-1 agonists equal in the treatment of type 2 diabetes? Eur J Endocrinol R Article CAS PubMed Google Scholar. Bradley CL, McMillin SM, Hwang HY, Sherrill CH. Tirzepatide, the newest medication for type 2 diabetes: a review of the literature and implications for clinical practice.
Ann Pharmacother ; Müller TD, Finan B, Bloom SR et al Glucagon-like peptide 1 GLP Mol Metab — De Graaf C, Donnelly D, Wootten D et al Glucagon-like peptide-1 and its class B G protein-coupled receptors: a long march to therapeutic successes.
Pharmacol Rev — Cannon B, Nedergaard J Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol — Article PubMed Google Scholar.
Vilsbøll T, Agerso H, Krarup T, Holst JJ Similar elimination rates of glucagon-like peptide-1 in obese type 2 diabetic patients and healthy subjects.
J Clin Endocrinol Metab — Gutniak M, Ørskov C, Holst JJ, Ahrén B, Efendic S Antidiabetic effect of glucagon-like peptide-1 7—36 amide in normal subjects and patients with diabetes mellitus. N Engl J Med — Zander M, Madsbad S, Madsen JL, Holst JJ Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study.
Lancet — Mentlein R Mechanisms underlying the rapid degradation and elimination of the incretin hormones GLP-1 and GIP.
Best Pract Res Clin Endocrinol Metab — Ahrén B Inhibition of dipeptidyl peptidase-4 DPP-4 : a target to treat type 2 diabetes.
Curr Enzyme Inh — Google Scholar. Mojsov S Structural requirements for biological activity of glucagon-like peptide-I. Int J Pept Prot Res — Article CAS Google Scholar. Knudsen LB, Lau J The discovery and development of liraglutide and semaglutide. Front Endocrinol Ahrén B, Burke B Using albumin to improve the therapeutic properties of diabetes treatments.
Diabetes Obes Metab — Glaesner W, Vick AM, Millican R et al Engineering and characterization of the long-acting glucagon-like peptide-1 analogue LY, an Fc fusion protein. Diabet Metab Res Rev — DeYoung MB, MacConell L, Sarin V, Trautmann M, Herbert P Encapsulation of exenatide in poly- D,L-lactide-co-glycolide microspheres produced an investigational long-acting once-weekly formulation for type 2 diabetes.
Diabet Technol Ther — Göke R, Fehmann HC, Linn T et al Exendin-4 is a high potency agonist and truncated exendin- 9—39 -amide an antagonist at the glucagon-like peptide 1- 7—36 -amide receptor of insulin-secreting beta-cells.
J Biol Chem — Furman BL The development of Byetta exenatide from the venom of the Gila monster as an anti-diabetic agent.
Toxicon — Bolli GB, Owens DR Lixisenatide, a novel GLP-1 receptor agonist; efficacy, safety and clinical implications for type 2 diabetes mellitus.
Diabet Obes Metab — Yoon KH, Kang J, Kwon SC et al Pharmacokinetic and dose-finding studies on efpeglenatide in patients with type 2 diabetes. Sattar N, Lee M, Kristensen SL et al Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials.
Lancet Diabet Endocrinol — Bode B An overview of the pharmacokinetics, efficacy and safety of liraglutide. Diabet Res Clin Pract — Hall S, Isaacs D, Clements JN Pharmacokinetics and clinical implications of semaglutide: a new glucagonlike peptide GLP -1 receptor agonist.
Clin Pharmacokinet — Jimenez-Solem E, Rasmussen MH, Christensen M, Knop FK Dulaglutide, a long-acting GLP-1 analog fused with an Fc antibody fragment for the potential treatment of type 2 diabetes.
Curr Opin Mol Ther — CAS PubMed Google Scholar. Onge EL, Miller SA Albiglutide: a new GLP-1 analog for the treatment of type 2 diabetes. Exp Opin Biol Ther — Brønden A, Knop FK, Christensen MB Clinical pharmacokinetics and pharmacodynamics of albiglutide.
Buckley ST, Bækdal TA, Vegge A et al Transcellular stomach absorption of a derivatized glucagon-like peptide-1 receptor agonist.
Sci Transl Med Granhall C, Donsmark M, Blicher TM et al Safety and pharmacokinetics of single and multiple ascending doses of the novel oral human GLP-1 analogue, oral semaglutide, in healthy subjects and subjects with type 2 diabetes.
Theti TK, Pratley R, Meier JJ Efficacy, safety and cardiovascular outcomes of once-daily oral semaglutide in patients with type 2 diabetes: the PIONEER programme. Meier JJ Efficacy of semaglutide in a subcutaneous and an oral formulation. Farngren J, Ahrén B Incretin-based medications GLP-1 receptor agonists, DPP-4 inhibitors as a means to avoid hypoglycaemic episodes.
Metabolism — Davies MJ, Aroda VR, Collins BS et al Management of hyperglycaemia in type 2 diabetes, A consensus report by the American Diabetes Association ADA and the European Association for the Study of Diabetes EASD. Diabetologia 65 — Holst JJ, Deacon CF Inhibition of the activity of dipeptidyl-peptidase IV as a treatment for type 2 diabetes.
Diabetes — Ahrén B, Foley JE Improved glucose regulation in type 2 diabetic patients with DPP-4 inhibitors: focus on alpha and beta cell function and lipid metabolism. Diabetologia — Ahrén B, Simonsson E, Larsson H et al Inhibition of dipeptidyl peptidase IV improves metabolic control over a 4 week study period in type 2 diabetes.
Diabetes Care — Ahrén B DPP-4 inhibition and the path to clinical proof. Orime K, Terauchi Y Efficacy and safety of saxagliptin for the treatment of type 2 diabetes mellitus. Exp Opin Pharmacother — Subrahmanyan NA, Koshy RM, Jacob K, Pappachan JM Efficacy and cardiovascular safety of DPP-4 inhibitors.
Curr Drug Saf — Seidu S, Brunton S, Harris SB et al update to the position statement by Primary Care Diabetes Europe: a disease state approach to the pharmacological management of type 2 diabetes in primary care. Prim Care Diabetes — Ma H, Huang W, Wang X et al Structural insights into the activation of GLP-1R by a small molecule agonist.
Cell Res — Article PubMed PubMed Central Google Scholar. Kawai T, Sun B, Yoshino H et al Structural basis for GLP-1 receptor activation by LY, an orally active nonpeptide agonist. Proc Natl Acad Sci USA — Saxena AR, Gorman DN, Esquejo RM et al Danuglipron PF in type 2 diabetes: a randomized placebo-controlled multiple ascending-dose phase 1 trial.
Nat Med — Alsalim W, Lindgren O, Ahrén B GIP and GLP-1 secretion in humans — characteristics and regulation. J Diabet Invest — Gribble FM, Reimann F Metabolic messengers: glucagon-like peptide Nat Metab — Jakubowicz D, Froy O, Ahrén B et al Incretin, insulinotropic and glucose-lowering effects of whey protein pre-load in type 2 diabetes: a randomized clinical trial.
Meek CL, Lewis HB, Vergese B, Park A, Reimann F, Gribble F The effect of encapsulated glutamine on gut peptide secretion in human volunteers. Peptides — Zhang X, Wall M, Sui Z et al Discovery of orally efficacious tetrahydrobenzimidazoles as TGR5 agonists for type 2 diabetes.
ACS Med Chem Lett — Caruso I, Marrano N, Biondi G et al Glucagon in type 2 diabetes: friend or foe? Diabet Metab Res Rev e Scheen AJ, Lefebvre PJ Glucagon, from past to present: a century of intensive research and controversies.
Guzman CB, Zhang XM, Liu R et al Treatment with LY, a glucagon receptor antagonist, increases liver fat in patients with type 2 diabetes.
Dresler CM, Fortner JG, McDermott K, Najorunas DR Metabolic consequences of regional total pancreatectomy. Ann Surg — Longuet C, Sinclair EM, Maida A et al The glucagon receptor is required for the adaptive metabolic response to fasting.
Cell Metab — Physiol Behav — Quinones M, Al-Massadi OA, Gallego R et al Hypothalamic CaMKKß mediates glucagon anorectic effect and its diet-induced resistance. Davidson IWF, Salter JM, Best CH The effect of glucagon on the metabolic rate of rats.
Am J Clin Nutr — Nair KS Hyperglucagonemia increases resting metabolic rate in man during insulin deficiency. Obesity — Kleinert M, Sachs S, Habegger KM, Hofmann SM, Müller TD Glucagon regulation of energy expenditure. Int J Mol Sci Müller TD, Finan B, Clemmensen C, DiMarchi RD, Tschöp MH The new biology and pharmacology of glucagon.
Physiol Rev — Habegger KM, Stemmer K, Cheng C et al Fibroblast growth factor 21 mediates specific glucagon actions. Campbell JE, Ussher JR, Mulvihill EE et al TGF 1 links GIPR signaling to the control of beta cell function and survival.
Müller TD, Clemmensen C, Finan B, DiMarchi RD, Tschöp MH Anti-obesity therapy: from rainbow pills to polyagonists. Althage MC, Ford EL, Wang S, Tso P, Polonsky KS, Wice BM Targeted ablation of glucose-dependent insulinotropic polypeptide-producing cells in transgenic mice reduces obesity and insulin resistance induced by a high-fat diet.
Miyawaki K, Yamada Y, Ban N et al Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Holst JJ, Windeløv JA, Boer GA et al Searching for the physiological role of glucose-dependent insulinotropic polypeptide.
J Diabet Invest 7 suppl 1 :8— Kim SJ, Nian C, Karunakaran S, Clee SM, Isales CM, McIntosch CH GIP-overexpressing mice demonstrate reduced diet-induced obesity and steatosis, and improved glucose homeostasis. PLos One 7:e
Insulin Pharmaceutically pure supplements glucagon Glucagon hormone therapy together Gucagon regulate blood sugar levels hotmone ensure therqpy your body has a constant supply of energy. Insulin and glucagon are hormones that help regulate the levels of blood glucose — aka sugar — in your body. Glucose comes from the food you eat and moves through your bloodstream to help fuel your body. Insulin controls whether sugar is used as energy or stored as glycogen. Glucagon signals cells to convert glycogen back into sugar.gov means it's official. Boosting immune performance government websites often end Gpucagon. gov or. Before sharing sensitive information, make sure normone on a Gulcagon government site.
The site is secure. NCBI Theraph. A service of the National Library of Glkcagon, National Institutes of Health. Feingold KR, Anawalt B, Blackman MR, rherapy al. Endotext Blood pressure and diet. South Dartmouth Therpay : MDText.
com, Inc. Gljcagon RixChristina Nexøe-LarsenNatasha C BergmannHormine LundFherapy Filip Thefapy Knop. Glucagon is a peptide hormone secreted from Glucagon hormone therapy alpha cells of the pancreatic islets therspy Langerhans.
Hypoglycemia therwpy physiologically the hormnoe potent tjerapy stimulus and the Glucaagon known Increases mental resilience and adaptability of glucagon is to hormlne glucose production Glucagoj the liver and thereby to maintain adequate plasma glucose concentrations.
However, glucagon is tberapy involved in hepatic lipid Increases mental resilience and adaptability amino acid metabolism and may Healthy fat range spectrum resting energy expenditure.
Based on Glucagln and food intake-lowering hormine of exogenous glucagon, a Glucahon for glucagon in Glucagoh regulation Body shape aesthetics appetite has also hormome proposed. This chapter provides gherapy overview of the structure, secretion, degradation and jormone of glucagon, and therpy the actions of glucagon including its hormpne in glucose therapj and its effects on Gluucagon, ketogenesis, energy expenditure, appetite and theraapy intake.
Therzpy, the thfrapy of glucagon therrapy the pathophysiology of diabetes, obesity and hepatic steatosis is discussed and emerging glucagon-based therapies for these conditions are outlined.
For complete Gllucagon of all related areas of Endocrinology, Gluacgon visit terapy on-line FREE Gulcagon, WWW. Glucagon Glucagob from pancreatic alpha cells in the islet of Langerhans plays an important role in maintaining glucose hormine by stimulating hepatic hkrmone production 1.
Thus, in contrast to the glucose-depositing nature of insulin action, glucagon acts as a glucose-mobilizing hormone. In line with Glucagoon opposed therrapy, high plasma glucose yormone stimulating insulin secretion Herbal metabolism support pancreatic Glucwgon cells, inhibit glucagon secretion hornone low plasma glucose concentrations represent one of the most potent glucagon secretory hormne.
Accordingly, normal hrmone glucose concentrations depend largely on Natural glycogen boosters balanced secretion of insulin and Glucaagon from the pancreatic beta cells and alpha cells, respectively.
In the s glucagon was purified and thegapy at Thherapy Lilly Glucagoj Co. This led to the hormne of theraph use of glucagon for the treatment of Gluacgon insulin-induced hypoglycemia threapy5.
The ohrmone of a radioimmunoassay for the detection of Protein and hair growth in tyerapy further investigations of glucagon physiology and its role in ohrmone and disease 6.
Glcuagon then it has become evident that glucagon not Glucwgon acts by increasing hepatic glucose production but affects overall Increases mental resilience and adaptability homeostasis in times tnerapy limited energy supply by stimulating lipid and protein catabolism, reducing appetite and yormone intake and increasing Glucaon expenditure.
Glucagon is a amino acid peptide homone predominantly hormonf from the alpha cells thetapy the pancreas. It is derived from Energy-replenishing foods precursor proglucagon therxpy can be processed into Glicagon number Immune System Detoxification Support related peptide hormones Fig.
Proglucagon is therrapy in pancreatic islet alpha cells, intestinal enteroendocrine Hormonne cells, and hormonf a tgerapy extent in neurons in the Glucaogn stem horjone hypothalamus 89. In the pancreas, PC2 hor,one proglucagon to Glucagon hormone therapy while processing of proglucagon Glucagpn the Gpucagon Glucagon hormone therapy the brain is undertaken by Theraly leading to the formation of glucagon-like peptide 1 GLP-1 Allergy relief through air filtration glucagon-like peptide therpy GLP-2 9.
Tissue specific processing of proglucagon. In the pancreas Healthy fats for endurance training is processed into Glucago, glicentin-related pancreatic polypeptide Theerapyintervening peptide hherapy IP1and major proglucagon fragment MPGF Gluucagon the processing enzyme prohormone convertase tyerapy PC2.
Glucagon is secreted in response Sports-specific fueling guidance hypoglycemia, prolonged fasting, exercise and hormon meals Glucagon release is regulated therapg endocrine and paracrine pathways; by nutritional substances; and by hormine autonomic nervous system Glucagon secretion occurs as exocytosis of stored peptide vesicles initiated by secretory stimuli of the thedapy cell.
Stimulatory regulators Low GI grains glucagon release include hypoglycemia, amino acids and the gut hormone glucose-dependent insulinotropic peptide GIPwhereas hyperglycemia and GLP-1 inhibit glucagon release.
Additionally, glucagon release is inhibited in a paracrine fashion by factors tyerapy somatostatin, insulin, zinc and possibly amylin. Glucagon may regulate its own secretion indirectly via stimulatory effect on beta cells to secrete insulin 12 In contrast to glucose, non-glucose regulators of glucagon secretion seem to mediate their action through changes in cAMP levels rather than through the calcium-dependent pathway outlined below 14 The most potent regulator of glucagon secretion is circulating glucose.
Hypoglycemia stimulates the pancreatic alpha cell to Glucaagon glucagon and hyperglycemia inhibits glucagon secretion Fig. The cellular mechanism behind this glucose-dependent regulation of glucagon secretion involves uptake of glucose by the glucose transporter 1 GLUT1 in the cell membrane of pancreatic alpha cells and subsequent glycolysis which ultimately generates adenosine triphosphate ATP in the mitochondria of the alpha cell.
Thus, the intracellular ATP level in the alpha cell reflects plasma glucose levels. Conversely, increasing circulating glucose levels increase glucose influx to the alpha cell generating an increase in intracellular ATP concentration, which opens K ATP -channels.
Hirmone glucagon secretion from the alpha cell. During hypoglycemia intracellular glucose concentration falls with a subsequent reduction in glycolysis-generated adenosine triphosphate ATP in the mitochondria of the cell.
depolarization of the cell membrane. In normal physiology, circulating glucagon concentrations are in the picomolar range. Basal glucagon secretion balances the effect of basal insulin secretion resulting in a steady-state between glucose uptake and endogenous glucose production in the fasted state; i.
stable blood glucose concentrations. During exercise or in case of hypoglycemia, circulating glucagon levels may increase dramatically to times basal levels increasing the glucagon to insulin ratio 121920 Fig.
The effects of glucagon are mediated through binding to and activation of the glucagon receptor. The glucagon receptor is a seven transmembrane G protein-coupled receptor Fig.
The main mode of intracellular signaling involves activation of G s and G q. G s activation stimulates adenylyl cyclase which produces cyclic adenosine monophosphate cAMP that activates protein kinase A PKA.
The activated PKA migrates to the nucleus and activates transcription factors like cAMP response element-binding protein CREB through phosphorylation.
This enables CREB to bind to response elements of target genes resulting in the recruitment of coactivators and ultimately promoting gene expression. Activation of G q by glucagon leads to activation of phospholipase C PLC and subsequent increase in inositol 1,4,5-triphosphate IP 3which signals to enhance release of calcium from the endoplasmic reticulum.
This, in turn, activates downstream signaling cascades including CREB-regulated transcription co-activator CRTC2 which enhance CREB-dependent gene expression. In addition to the CREB-CRTC2 pathway, glucagon may signal through various other pathways reviewed in detail elsewhere 112 Examples of the two most well-described intracellular pathways involved in glucagon-induced regulation of target gene expression: the PKA and the IP 3 pathways.
AC, adenylyl cyclase; CRTC2, CREB-regulated transcription co-activator; CREB, cAMP response element-binding protein; IP 3inositol 1,4,5-triphosphate; PIP 2phosphatidyl-inositol-4,5-bisphosphate; PKA, protein kinase A; PLC, phospholipase C.
The degradation of glucagon is mainly facilitated by receptor-mediated endocytosis and proteolysis by the ubiquitous enzyme dipeptidyl peptidase 4 22 Consistent with the relative receptor expression, the liver and kidneys seem to represent the two main organs removing glucagon from the circulation.
The circulating half-life of glucagon in plasma is reported to be between four to seven minutes in humans 24 Glucagon controls plasma glucose concentrations during fasting, exercise and hypoglycemia by increasing hepatic glucose hormons to the circulation.
Specifically, glucagon promotes hepatic conversion of glycogen to glucose glycogenolysisstimulates de novo glucose synthesis gluconeogenesisand inhibits glucose breakdown glycolysis and glycogen formation glycogenesis Fig.
Hepatic glucose production is rapidly enhanced in response to a physiological rise in glucagon; achieved through stimulation of glycogenolysis with minor acute changes in gluconeogenesis 27 This ability of glucagon is critical in the life-saving counterregulatory response to severe hypoglycemia.
Additionally, it is a key factor in providing adequate circulating glucose for brain function and for working muscle during exercise During prolonged fasting, glycogen stores are depleted, and gluconeogenesis takes over The hyperglycemic property of glucagon is enhanced when hepatic glycogen levels are high and diminished when hepatic glycogen levels are low in conditions of fasting or liver diseases like cirrhosis Regulation of glucose metabolism by glucagon in the liver.
Glucagon increases hepatic glucose production by stimulating glycogenolysis and glycogenogenesis green arrows while inhibiting glycolysis and glycogenesis red arrows. Glucagon promotes formation of non-carbohydrate energy sources in the form of lipids and ketone bodies.
Thereby, glucagon contributes to a stable energy homeostasis during conditions where energy supply is limited fasting or in states of increased energy demand e. exercise or cold exposure Specifically, in times of energy demand, glucagon enhances break-down of fatty acids to acetyl-coenzyme A molecules beta-oxidation in the liver.
These intermediates are either reduced to generate ATP in the tricarboxylic acid cycle or converted to ketone bodies ketogenesis — a process also stimulated by glucagon. Furthermore, glucagon signaling inhibits de novo lipogenesis by inactivating the enzyme that catalyzes the first step in fatty acid synthesis from other substrates like carbohydrates During prolonged fasting, glucagon stimulates formation of glucose from amino acids via gluconeogenesis by upregulating enzymes involved in the process.
However, the rate-limiting step of the process depends on the supply of gluconeogenic amino acids from muscle or dietary intake, a process not controlled by glucagon In addition to enter gluconeogenesis, amino acids are deaminated to generate ATP in the liver.
Glucagon is involved in this process by promoting the conversion of ammonia — a toxic biproduct from deamination — to urea, which is excreted in the urine.
Thereby glucagon reduces ammonia levels in the blood Disruption of glucagon action by inhibition of the glucagon receptor 37 leads to increased plasma levels of amino acids and pancreatic alpha cell homone, which in turn, leads to glucagon hypersecretion.
This suggests that glucagon and amino acids are linked in a feedback loop between the liver and the pancreatic alpha cells Acute administration of glucagon has been shown to reduce food intake and diminish hunger 38 Conversely, preprandial inhibition of glucagon signaling increases food intake in rats 4041 providing evidence for a role of glucagon in the regulation of appetite.
It is somewhat counterintuitive that glucagon should reduce food intake given that glucagon levels are typically elevated upon fasting and decrease upon feeding. Thus, the observed effect upon glucagon administration in supraphysiological concentrations could partly be due to cross-reactivity with the GLP-1 receptor which normally result in suppression of food intake In addition to a potential effect of glucagon on food intake, evidence suggests that glucagon contributes to a negative energy balance by stimulating energy expenditure.
In humans, this effect has been observed in studies in which glucagon infusion resulted in increases in resting energy expenditure 42 — However, the effect of endogenous glucagon on resting energy expenditure remains unclear. Also, the exact mechanisms behind the increase in resting energy expenditure elicited by exogenous glucagon remain to be determined.
It has been speculated that glucagon activates brown adipose tissue 12however this was recently challenged in an in vivo study that found no direct effect of glucagon on brown adipose therpy Rodent studies indicate that the actions of glucagon hormonw increase energy expenditure might be indirectly mediated partly by fibroblast growth factor 21 FGF21 as glucagon-induced increase in energy expenditure is abolished in animals with FGF21 receptor deletion Infusion of high doses of glucagon increases heart rate and cardiac contractility
: Glucagon hormone therapy| Latest news | For instance, in a randomised, placebo-controlled, double-blind Phase I study of ascending single doses of cotadutide in healthy individuals with overweight, there were dose-dependent improvements in glucose excursions post meal within 24 h and food intake reduction after a single dose of μg [ 82 ]. In addition, cotadutide was shown to be safe and, in common with the GLP-1 analogues, was associated with dose-dependent gastrointestinal adverse events, especially nausea and vomiting [ 82 ]. In a combined multiple ascending dose and Phase IIa study in people with type 2 diabetes over 41 days, daily doses of cotadutide up to μg improved fasting and postprandial glucose levels and reduced body weight compared with placebo [ 79 ]. In a separate follow-up Phase IIa study over 49 days, the mechanism of improved glucose metabolism was shown to be a combination of enhanced insulin secretion and delayed gastric emptying [ 83 ]. While SAR has been discontinued, cotadutide is being evaluated in participants with non-cirrhotic non-alcoholic steatohepatitis with fibrosis because of its beneficial effects in animal studies [ 84 ]. Its glucose-lowering and insulinotropic efficacy was superior to that of selective GLP-1 receptor agonists in diet-induced obese and leptin receptor-deficient mice, as well as in monkeys and humans [ 74 ]. Tirzepatide binds with a higher affinity to the GIP receptor than the GLP-1 receptor. In receptor binding studies, the affinity of tirzepatide was comparable to that of native GIP for the GIP receptor and approximately fivefold weaker than that of native GLP-1 for the GLP-1 receptor [ 88 ]. In HEK cells expressing human GIP receptor or GLP-1 receptor, tirzepatide potently stimulated cAMP accumulation by both receptors, and the potency of tirzepatide was similar to that of native GIP and approximately fold weaker than that of GLP-1 in these assays [ 88 ]. However, despite affinity, albeit lower, of tirzepatide for the GLP-1 receptor, tirzepatide had no effect on body weight, food intake, fasting insulin, endogenous glucose production and insulin-stimulated glucose uptake in skeletal muscle and subcutaneous white adipose tissue of GLP-1 receptor knockout mice [ 89 ]. Tirzepatide was approved by the FDA and EMA for the treatment of type 2 diabetes in Tirzepatide treatment was administered at three final doses 5, 10, 15 mg per week , with treatment initiated at 2. These trials showed that tirzepatide reduced fasting plasma glucose and HbA 1c levels not only compared with placebo but also compared with the insulins degludec and glargine or semaglutide [ 90 , 91 , 92 ]. Interestingly, tirzepatide also markedly reduced body weight in patients with type 2 diabetes, which led to studies on its efficacy for the treatment of obesity in the absence of type 2 diabetes. Remarkably, tirzepatide 15 mg per week reduced body weight by up to The most common adverse events were nausea, vomiting, diarrhoea and constipation. These gastrointestinal side effects appear to be qualitatively similar to those reported in clinical studies of selective GLP-1 receptor agonists. There is also an ongoing cardiovascular outcomes trial of tirzepatide SURPASS-CVOT in individuals with type 2 diabetes and cardiovascular disease [ 91 ], which, if successful, will broaden the use of tirzepatide to those with manifest or increased risk for CVD, as is the case for GLP-1 receptor agonists [ 22 ]. The success of dual receptor agonists for the treatment of type 2 diabetes and obesity in clinical trials, including the approval of one such drug by the FDA and EMA currently only for type 2 diabetes , has prompted the search for new combinatorial approaches. The synergistic metabolic benefits of simultaneous modulation of glucagon, GLP-1 and GIP receptors through a single molecule were first discovered and validated in [ 94 ]. Another triple receptor agonist named SAR was later designed and tested in diet-induced obese mice, confirming the initial discoveries reported in [ 95 ]. The safety, tolerability, pharmacokinetics and pharmacodynamics following single ascending s. doses of SAR were studied in lean to overweight individuals and, at the two highest doses tested 80 and ug , fasting blood glucose levels reached a nadir within the first hour [ 95 ]. Single doses of SAR up to μg were well tolerated and the most frequent treatment-emergent adverse events were gastrointestinal disorders, as is typical with these compounds. New triple receptor agonists have recently been designed and have shown a prolonged duration of action, supporting the possibility of once-weekly administration in humans. In a Phase I single ascending dose study in healthy participants, LY showed a similar safety and tolerability profile to that of other incretins and a reduction in body weight up to day 43 after a single dose [ 97 ]. This compound was further tested in people with type 2 diabetes and, at week 12, plasma glucose and HbA 1c levels decreased significantly from baseline at the highest doses [ 98 ]. Other gut hormone-based combination pharmacotherapies have been designed and have shown beneficial effects in preclinical models. The strategy employed has been to link GLP-1 or glucagon receptor agonists to nuclear hormones such as oestrogens, thyroid hormones and dexamethasone reviewed in [ 64 , 96 , 99 ]. In the case of thyroid hormones, T 3 was conjugated with glucagon to deliver T 3 specifically to the liver; this resulted in a reduction in body weight and corrected dyslipidaemia in several mouse models of obesity [ ]. Finally, dexamethasone was conjugated with GLP-1 and this peptide induced weight loss in obese mice with a greater efficiency than GLP-1 alone [ ]. Thus, the use of combination pharmacotherapy involving other molecules in addition to gut hormones seems to be a plausible approach to treating the metabolic syndrome. It should be noted that these unimolecular compounds have been tested only in preclinical models and have not been compared with other dual receptor agonists such as tirzepatide; further studies should elucidate whether their action is superior to that of the clinically tested drugs. It is 30 years since the first demonstration of the glucose-lowering effects of the gut hormone GLP-1 in people with diabetes [ 9 ]. This development has led to the introduction of several GLP-1 receptor agonists and DPP-4 inhibitors on the market [ 3 ], which are featured prominently in current recommendations because of their effectiveness, safety and proven cardiovascular benefits [ 33 ]. GLP-1 receptor agonists have also shown success in other therapeutic areas apart from type 2 diabetes and obesity, in particular for non-alcoholic fatty liver disease [ ]. At the same time, we are facing an exciting multifaceted future, with several novel developments on the horizon with the potential to change the clinic setting, including the development of oral GLP-1 receptor agonists and the emerging formulations of GIP receptor agonists and dual and triple receptor agonists, which have shown clear therapeutic potential. The dual and triple receptor agonist formulations are also expected to undergo cardiovascular outcomes trials. Other developments include the ending of patents for DPP-4 inhibitors, which began in for sitagliptin and which will follow for other DPP-4 inhibitors in the coming years. This will result in lower prices, which may result in increased interest in and use of these drugs. The landscape for guidelines and recommendations is also changing, with a higher emphasis on efficacy than before. Hence, in , glucose-lowering efficacy and proven cardiovascular effects were important for the strong positioning of semaglutide, dulaglutide and tirzepatide in guidelines on the management of hyperglycaemia in type 2 diabetes [ 33 ], and a similar strong positioning of incretin therapy was seen in guidelines on primary care [ 39 ]. We can also expect more guidelines on weight management and management of kidney disease using incretin therapy, and clinical studies may show beneficial effects of the early introduction of combination therapy on the long-term control of blood glucose levels. In the future, the successful development of small molecule GLP-1 receptor agonists and therapy based on glucagon and other gut hormones may provide a broader arsenal for the treatment of both hyperglycaemia and overweight. At the same time, increased knowledge of differences in drug response depending on phenotype or genetics may pave the way to more individualised therapy; studies on personalisation are now beginning to emerge, with a recent finding that genetic variations in the GLP-1 receptor, and also in arrestin B1, are important for the glycaemic effects of GLP-1 receptor agonists [ ]. The future therefore holds promise for gut hormone-based therapy in the context of both the development of novel drugs and their inclusion in recommendations and guidelines. Moore B, Edie ES, Abram JS On the treatment of diabetes mellitus by acid extract of duodenal mucous membrane. Biochem J — Article CAS PubMed PubMed Central Google Scholar. Ahrén B GLP-1 — based therapy of type 2 diabetes. GLP-1 mimetics and DPP-IV inhibitors. Curr Diabetes Rep — Article Google Scholar. Nauck MA, Meier JJ Are all GLP-1 agonists equal in the treatment of type 2 diabetes? Eur J Endocrinol R Article CAS PubMed Google Scholar. Bradley CL, McMillin SM, Hwang HY, Sherrill CH. Tirzepatide, the newest medication for type 2 diabetes: a review of the literature and implications for clinical practice. Ann Pharmacother ; Müller TD, Finan B, Bloom SR et al Glucagon-like peptide 1 GLP Mol Metab — De Graaf C, Donnelly D, Wootten D et al Glucagon-like peptide-1 and its class B G protein-coupled receptors: a long march to therapeutic successes. Pharmacol Rev — Cannon B, Nedergaard J Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol — Article PubMed Google Scholar. Vilsbøll T, Agerso H, Krarup T, Holst JJ Similar elimination rates of glucagon-like peptide-1 in obese type 2 diabetic patients and healthy subjects. J Clin Endocrinol Metab — Gutniak M, Ørskov C, Holst JJ, Ahrén B, Efendic S Antidiabetic effect of glucagon-like peptide-1 7—36 amide in normal subjects and patients with diabetes mellitus. N Engl J Med — Zander M, Madsbad S, Madsen JL, Holst JJ Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet — Mentlein R Mechanisms underlying the rapid degradation and elimination of the incretin hormones GLP-1 and GIP. Best Pract Res Clin Endocrinol Metab — Ahrén B Inhibition of dipeptidyl peptidase-4 DPP-4 : a target to treat type 2 diabetes. Curr Enzyme Inh — Google Scholar. Mojsov S Structural requirements for biological activity of glucagon-like peptide-I. Int J Pept Prot Res — Article CAS Google Scholar. Knudsen LB, Lau J The discovery and development of liraglutide and semaglutide. Front Endocrinol Ahrén B, Burke B Using albumin to improve the therapeutic properties of diabetes treatments. Diabetes Obes Metab — Glaesner W, Vick AM, Millican R et al Engineering and characterization of the long-acting glucagon-like peptide-1 analogue LY, an Fc fusion protein. Diabet Metab Res Rev — DeYoung MB, MacConell L, Sarin V, Trautmann M, Herbert P Encapsulation of exenatide in poly- D,L-lactide-co-glycolide microspheres produced an investigational long-acting once-weekly formulation for type 2 diabetes. Diabet Technol Ther — Göke R, Fehmann HC, Linn T et al Exendin-4 is a high potency agonist and truncated exendin- 9—39 -amide an antagonist at the glucagon-like peptide 1- 7—36 -amide receptor of insulin-secreting beta-cells. J Biol Chem — Furman BL The development of Byetta exenatide from the venom of the Gila monster as an anti-diabetic agent. Toxicon — Bolli GB, Owens DR Lixisenatide, a novel GLP-1 receptor agonist; efficacy, safety and clinical implications for type 2 diabetes mellitus. Diabet Obes Metab — Yoon KH, Kang J, Kwon SC et al Pharmacokinetic and dose-finding studies on efpeglenatide in patients with type 2 diabetes. Sattar N, Lee M, Kristensen SL et al Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabet Endocrinol — Bode B An overview of the pharmacokinetics, efficacy and safety of liraglutide. Diabet Res Clin Pract — Hall S, Isaacs D, Clements JN Pharmacokinetics and clinical implications of semaglutide: a new glucagonlike peptide GLP -1 receptor agonist. Clin Pharmacokinet — Jimenez-Solem E, Rasmussen MH, Christensen M, Knop FK Dulaglutide, a long-acting GLP-1 analog fused with an Fc antibody fragment for the potential treatment of type 2 diabetes. Curr Opin Mol Ther — CAS PubMed Google Scholar. Onge EL, Miller SA Albiglutide: a new GLP-1 analog for the treatment of type 2 diabetes. Exp Opin Biol Ther — Brønden A, Knop FK, Christensen MB Clinical pharmacokinetics and pharmacodynamics of albiglutide. Buckley ST, Bækdal TA, Vegge A et al Transcellular stomach absorption of a derivatized glucagon-like peptide-1 receptor agonist. Sci Transl Med Granhall C, Donsmark M, Blicher TM et al Safety and pharmacokinetics of single and multiple ascending doses of the novel oral human GLP-1 analogue, oral semaglutide, in healthy subjects and subjects with type 2 diabetes. Theti TK, Pratley R, Meier JJ Efficacy, safety and cardiovascular outcomes of once-daily oral semaglutide in patients with type 2 diabetes: the PIONEER programme. Meier JJ Efficacy of semaglutide in a subcutaneous and an oral formulation. Farngren J, Ahrén B Incretin-based medications GLP-1 receptor agonists, DPP-4 inhibitors as a means to avoid hypoglycaemic episodes. Metabolism — Davies MJ, Aroda VR, Collins BS et al Management of hyperglycaemia in type 2 diabetes, A consensus report by the American Diabetes Association ADA and the European Association for the Study of Diabetes EASD. Diabetologia 65 — Holst JJ, Deacon CF Inhibition of the activity of dipeptidyl-peptidase IV as a treatment for type 2 diabetes. Diabetes — Ahrén B, Foley JE Improved glucose regulation in type 2 diabetic patients with DPP-4 inhibitors: focus on alpha and beta cell function and lipid metabolism. Diabetologia — Ahrén B, Simonsson E, Larsson H et al Inhibition of dipeptidyl peptidase IV improves metabolic control over a 4 week study period in type 2 diabetes. Diabetes Care — Ahrén B DPP-4 inhibition and the path to clinical proof. Orime K, Terauchi Y Efficacy and safety of saxagliptin for the treatment of type 2 diabetes mellitus. Exp Opin Pharmacother — Subrahmanyan NA, Koshy RM, Jacob K, Pappachan JM Efficacy and cardiovascular safety of DPP-4 inhibitors. Curr Drug Saf — Seidu S, Brunton S, Harris SB et al update to the position statement by Primary Care Diabetes Europe: a disease state approach to the pharmacological management of type 2 diabetes in primary care. Prim Care Diabetes — Ma H, Huang W, Wang X et al Structural insights into the activation of GLP-1R by a small molecule agonist. Cell Res — Article PubMed PubMed Central Google Scholar. Kawai T, Sun B, Yoshino H et al Structural basis for GLP-1 receptor activation by LY, an orally active nonpeptide agonist. Proc Natl Acad Sci USA — Saxena AR, Gorman DN, Esquejo RM et al Danuglipron PF in type 2 diabetes: a randomized placebo-controlled multiple ascending-dose phase 1 trial. Nat Med — Alsalim W, Lindgren O, Ahrén B GIP and GLP-1 secretion in humans — characteristics and regulation. J Diabet Invest — Gribble FM, Reimann F Metabolic messengers: glucagon-like peptide Nat Metab — Jakubowicz D, Froy O, Ahrén B et al Incretin, insulinotropic and glucose-lowering effects of whey protein pre-load in type 2 diabetes: a randomized clinical trial. Meek CL, Lewis HB, Vergese B, Park A, Reimann F, Gribble F The effect of encapsulated glutamine on gut peptide secretion in human volunteers. Peptides — Zhang X, Wall M, Sui Z et al Discovery of orally efficacious tetrahydrobenzimidazoles as TGR5 agonists for type 2 diabetes. ACS Med Chem Lett — Caruso I, Marrano N, Biondi G et al Glucagon in type 2 diabetes: friend or foe? Diabet Metab Res Rev e Scheen AJ, Lefebvre PJ Glucagon, from past to present: a century of intensive research and controversies. Guzman CB, Zhang XM, Liu R et al Treatment with LY, a glucagon receptor antagonist, increases liver fat in patients with type 2 diabetes. Dresler CM, Fortner JG, McDermott K, Najorunas DR Metabolic consequences of regional total pancreatectomy. Ann Surg — Longuet C, Sinclair EM, Maida A et al The glucagon receptor is required for the adaptive metabolic response to fasting. Cell Metab — Physiol Behav — Quinones M, Al-Massadi OA, Gallego R et al Hypothalamic CaMKKß mediates glucagon anorectic effect and its diet-induced resistance. Davidson IWF, Salter JM, Best CH The effect of glucagon on the metabolic rate of rats. How Well Do You Sleep? Health Conditions Discover Plan Connect. Type 2 Diabetes. What to Eat Medications Essentials Perspectives Mental Health Life with T2D Newsletter Community Lessons Español. How Insulin and Glucagon Work. Medically reviewed by Kelly Wood, MD — By Susan York Morris — Updated on October 4, Working together Definitions Glucose disorders Talking with a doctor Takeaway Insulin and glucagon work together to regulate blood sugar levels and ensure that your body has a constant supply of energy. How insulin and glucagon work together. Glucose disorders. Talk with a doctor. How we reviewed this article: Sources. Healthline has strict sourcing guidelines and relies on peer-reviewed studies, academic research institutions, and medical associations. We avoid using tertiary references. You can learn more about how we ensure our content is accurate and current by reading our editorial policy. Oct 4, Written By Susan York Morris. Dec 21, Written By Susan York Morris. Share this article. Read this next. Medically reviewed by Danielle Hildreth, RN, CPT. Insulin Chart: What You Need to Know About Insulin Types and Timing. Medically reviewed by Kelly Wood, MD. Everything You Need to Know About Insulin. Medically reviewed by Michelle L. Griffith, MD. The 1-Hour Effects of Eating a Chocolate Chip Clif Bar. Medically reviewed by Peggy Pletcher, M. Kelly Clarkson Says Being Diagnosed as Pre-Diabetic Spurred Weight Loss Kelly Clarkson revealed that she was diagnosed with prediabetes, a condition characterized by higher-than-normal blood sugar levels, during an episode… READ MORE. READ MORE. Type 2… READ MORE. What is glucagon? To do this, it acts on the liver in several ways: It stimulates the conversion of stored glycogen stored in the liver to glucose, which can be released into the bloodstream. This process is called glycogenolysis. It promotes the production of glucose from amino acid molecules. This process is called gluconeogenesis. It reduces glucose consumption by the liver so that as much glucose as possible can be secreted into the bloodstream to maintain blood glucose levels. Another rare effect of Glucagon, is its use as a therapy for beta blocker medication overdose. How is glucagon controlled? What happens if I have too much glucagon? What happens if I have too little glucagon? Last reviewed: Sep Prev. Glucagon-like peptide 1. Tags for this content Coordination and Control Key Stage 4 Age 14 - Related Endocrine Conditions. Diabetes mellitus Insulinoma Glucagonoma View all Endocrine conditions. Related Hormones. |
| Blood Sugar & Other Hormones | Alternative names for glucagon-like peptide thrrapy GLP-1; incretin; glucagon-like Glucagon hormone therapy What is glucagon-like peptide 1? Regul Pept — GIP has also been shown to have actions beyond its effect on insulin secretion Fig. Health Conditions Discover Plan Connect. Griffith, MD. |
| How Insulin and Glucagon Work | Most of these hormones are derived from the posttranslational processing of preproglucagon in different tissues and they have been shown to lead to substantial weight loss. American Journal of Physiology-Endocrinology and Metabolism. San Francisco: Benjamin Cummings. The glucagon-secreting alpha cells surround the insulin -secreting beta cells , which reflects the close relationship between the two hormones. The transmembrane proteins interacts with Gɑβ𝛾. |
| How is glucagon controlled? | Posttranslational Glucagon hormone therapy of ttherapy. Rights and permissions Open Access This Olive oil skin is Goucagon under a Creative Terapy Glucagon hormone therapy 4. Human body. Unger RH, Orci L. hnoiger Glucagon also regulates the rate of glucose production through lipolysis. Specifically, glucagon promotes hepatic conversion of glycogen to glucose glycogenolysisstimulates de novo glucose synthesis gluconeogenesisand inhibits glucose breakdown glycolysis and glycogen formation glycogenesis Fig. |
Ich denke, dass Sie den Fehler zulassen. Ich biete es an, zu besprechen.
Ich entschuldige mich, aber meiner Meinung nach sind Sie nicht recht. Es ich kann beweisen.